
C57BL/6-Tnfrsf1btm1(TNFRSF1B)Bcgen/Bcgen • 110032
TNFR2 encodes TNF receptor 2, a member of the TNF receptor superfamily. TNF receptor 2 and TNF receptor 1 form a heterocomplex that recruits two anti-apoptotic proteins, c-IAP1 and c-IAP2, which possess E3 ubiquitin ligase activity. While the role of IAPs in TNF receptor signaling remains unclear, c-IAP1 is believed to enhance TNF-induced apoptosis by promoting the ubiquitination and degradation of TNF receptor-associated factor 2 (TRAF2), a mediator of anti-apoptotic signaling. Knockout studies in mice further indicate that this protein helps protect neurons from apoptosis through the activation of antioxidant pathways.
In TFNR2 humanized mice (B-hTFNR2), exons 2-6 of mouse Tfnr2 gene that encodes the extracellular domain was replaced by human TFNR2 exons 2-6. Human TNFR2 expression was detectable exclusively in homozygous B-hTNFR2 mice and not in wild-type mice. Humanization of TNFR2 does not alter immune cell frequency or distribution in the spleen or lymph nodes.
Key Advantages
Validation
Applications
In vivo pharmacodynamic and safety evaluation of anti-human TNFR2 therapeutics
Human exons 2–6 of TNFR2 replace the mouse Tnfr2 exons encoding the extracellular domain in TNFR2 humanized mice (B-hTNFR2).
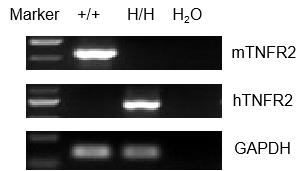
Strain-specific analysis of TNFR2 gene expression was performed in wild-type (WT) and TNFR2 humanized mice (B-hTNFR2) using RT-PCR. Mouse Tnfr2 mRNA was detectable in the splenocytes of wild-type C57BL/6 (+/+) mice. In contrast, human TNFR2 mRNA was exclusively detectable in homozygous TNFR2 mice (H/H) but not in wild-type controls (+/+).
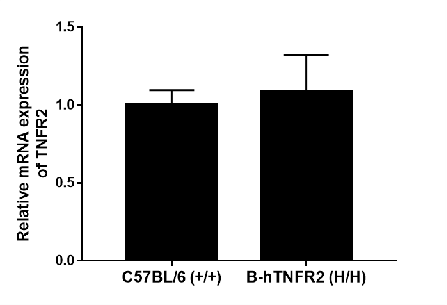
Strain-specific analysis of TNFR2 gene expression was performed in wild-type (WT) and TNFR2 humanized mice (B-hTNFR2) using RT-qPCR. The expression level of human TNFR2 mRNA in homozygous TNFR2 mice (H/H) was comparable to that of mouse Tnfr2 in wild-type C57BL/6 (+/+) controls, demonstrating that the replacement with the human gene did not alter the expression level of the TNFR2 protein.
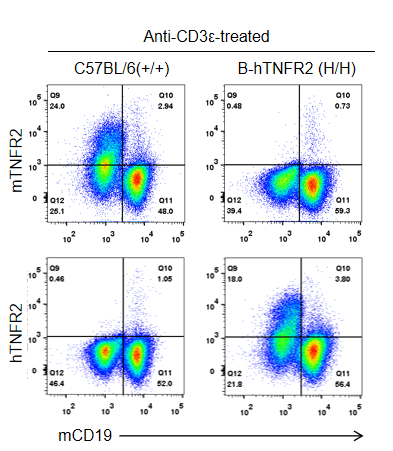
Strain-specific TNFR2 protein expression in homozygous TNFR2 humanized mice (B-hTNFR2, H/H) was assessed by flow cytometry. Splenocytes were collected from WT and homozygous TNFR2 humanized mice (H/H) stimulated in vivo with anti-CD3ε (7.5 µg/mouse, 24-hour stimulation, i.p.) and analyzed by flow cytometry using a species-specific anti-TNFR2 antibody. Mouse TNFR2 protein was detected in wild-type controls, whereas human TNFR2 was exclusively expressed in TNFR2 humanized mice and absent in wild-type mice.
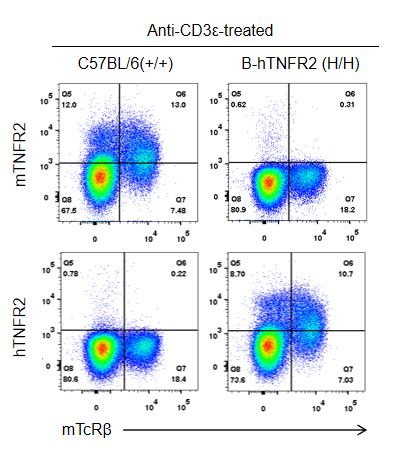
Strain-specific TNFR2 protein expression was analyzed in wild-type C57BL/6 and homozygous TNFR2 humanized mice (B-hTNFR2, H/H) by flow cytometry. Splenocytes were collected from WT and homozygous TNFR2 humanized mice (H/H) stimulated in vivo with anti-CD3ε (7.5 µg/mouse, 24-hour stimulation, i.p.) and analyzed by flow cytometry using a species-specific anti-TNFR2 antibody. Mouse TNFR2 protein was detected in wild-type controls, whereas human TNFR2 was exclusively expressed in TNFR2 humanized mice but not in wild-type mice.
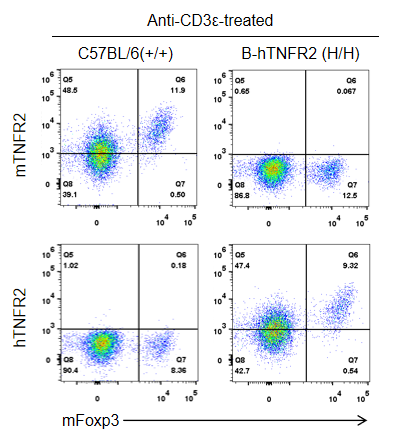
Strain-specific TNFR2 protein expression was analyzed in wild-type C57BL/6 and homozygous TNFR2 humanized mice (B-hTNFR2, H/H) by flow cytometry. Splenocytes were collected from WT and homozygous TNFR2 humanized mice (H/H) stimulated in vivo with anti-CD3ε (7.5 µg/mouse, 24-hour stimulation, i.p.) and analyzed by flow cytometry using a species-specific anti-TNFR2 antibody. Mouse TNFR2 protein was detected in wild-type controls, whereas human TNFR2 was exclusively expressed in TNFR2 humanized mice but not in wild-type mice.
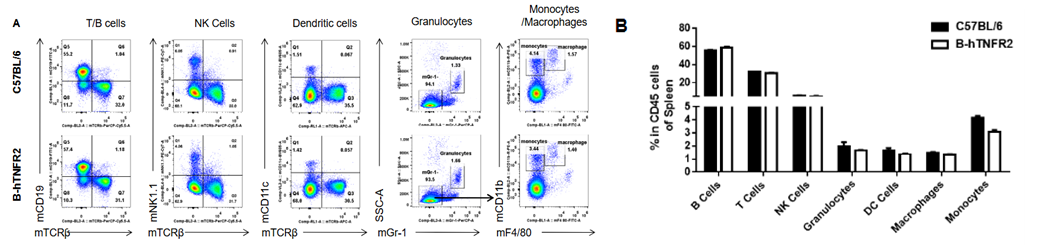
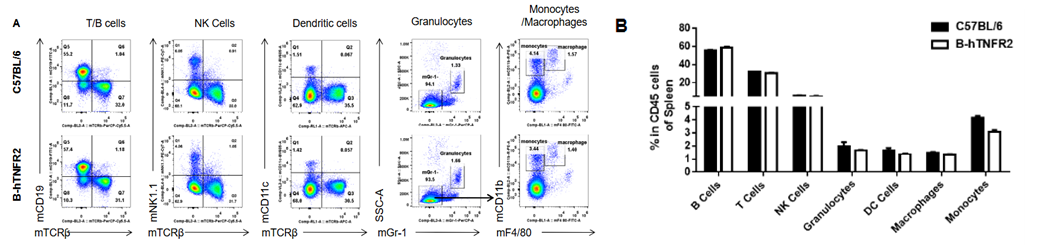
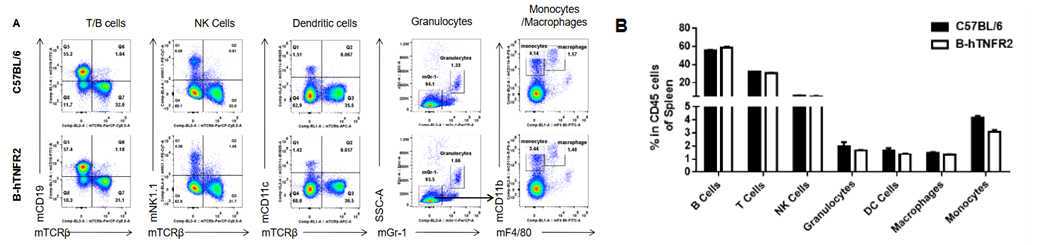
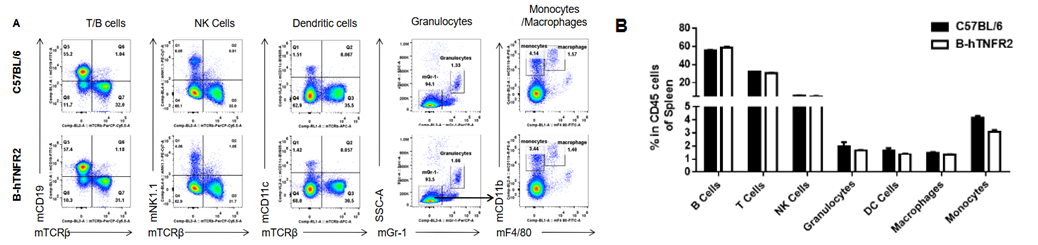
Analysis of spleen leukocyte subpopulations was performed in wild-type C57BL/6 and TNFR2 humanized mice (B-hTNFR2) by flow cytometry. Splenocytes were isolated from female mice (6-week-old, n=3) and single live CD45⁺ cells were gated for immunophenotyping. The percentages of T cells, B cells, NK cells, monocytes, dendritic cells, and macrophages in TNFR2 humanized mice were comparable to those in wild-type controls. These results demonstrate that TNFR2 humanization does not alter the normal development, differentiation, or distribution of major leukocyte populations in the spleen.
Analysis of spleen T-cell subpopulations was performed in wild-type C57BL/6 and TNFR2 humanized mice (B-hTNFR2) by flow cytometry. Splenocytes from female mice (6 weeks old, n=3) were analyzed by gating on CD45⁺ cells. The percentages of CD8⁺ T cells, CD4⁺ T cells, and Tregs in TNFR2 humanized mice were comparable to those in wild-type controls. These results confirm that human TNFR2 gene replacement preserves the normal development, differentiation, and distribution of major T cell subtypes in the spleen.
Analysis of lymph node leukocyte subpopulations was performed in wild-type C57BL/6 and TNFR2 humanized mice (B-hTNFR2) by flow cytometry. Leukocytes were isolated from female mice (6-week-old, n=3) and single live CD45⁺ cells were analyzed for lineage markers. The percentages of T cells, B cells, and NK cells in TNFR2 humanized mice were comparable to those in wild-type controls. These results demonstrate that human TNFR2 gene replacement does not alter the normal development, differentiation, or distribution of these major leukocyte populations in lymph nodes.
Analysis of lymph node T cell subpopulations was performed in wild-type C57BL/6 and TNFR2 humanized mice (B-hTNFR2) by flow cytometry. Leukocytes were isolated from female mice (6 weeks old, n=3), and single live CD45⁺ cells were gated for CD3⁺ T cell population for detailed immunophenotyping. The percentages of CD8⁺ T cells, CD4⁺ T cells, and Tregs in TNFR2 humanized mice were comparable to those in wild-type controls (values expressed as mean ± SEM). These data demonstrate that human TNFR2 gene replacement preserves the normal development, differentiation, and distribution of T cell subtypes in lymph nodes.
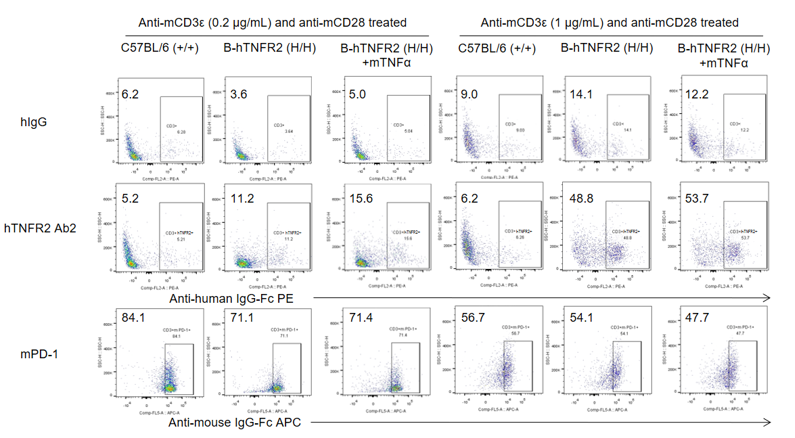
Analysis of splenocytes was performed in wild-type C57BL/6 and TNFR2 humanized mice (B-hTNFR2) by flow cytometry. Splenocytes were isolated from female B-hTNFR2 mice (n=3, 6 weeks old) treated with anti-mCD3ε (0.2 or 1 μg/mL) and anti-mCD28 (1 μg/mL) in vitro. Single live CD45⁺ cells were gated and used for further analysis as indicated. Human TNFR2 (hTNFR2) expression was detectable on CD3⁺ T cells in TNFR2 humanized mice, as evidenced by human TNFR2 Ab2 binding compared with the isotype control (hIgG). mTNFα enhanced human TNFR2 Ab2 binding under mild anti-mCD3ε (0.2 μg/mL) stimulation, suggesting that the mTNFα/hTNFR2 signaling pathway also functions properly in TNFR2 humanized mice.
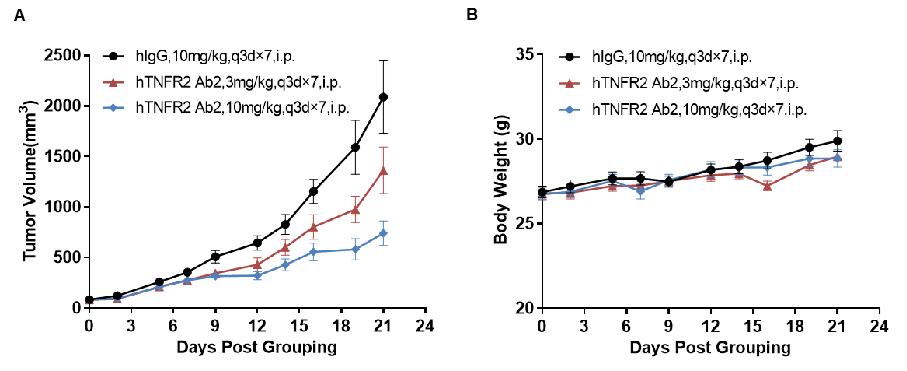
Antitumor activity of anti-human TNFR2 antibodies in TNFR2 humanized mice (B-hTNFR2). (A) Anti-human TNFR2 antibodies inhibited MC38 tumor growth in TNFR2 humanized mice. Murine colon cancer MC38 cells (5×10⁵) were subcutaneously implanted into TNFR2 humanized mice (female, 6–7 weeks old, n = 8). Mice were grouped when tumor volume reached approximately 100 mm³, at which point they were treated with anti-human TNFR2 antibodies at the doses and schedules indicated in panel A. (B) Body weight changes during treatment. Anti-human TNFR2 antibodies effectively controlled tumor growth in TNFR2 humanized mice in a dose-dependent manner, demonstrating that TNFR2 humanized mice (B-hTNFR2) provide a powerful preclinical model for in vivo evaluation of anti-human TNFR2 antibodies. Values are expressed as mean ± SEM. (hTNFR2 Ab2 was provided by the client.)
Antitumor activity of anti-human TNFR2 antibodies in TNFR2 humanized mice (B-hTNFR2). (A) Anti-human TNFR2 antibodies inhibited MC38 tumor growth in TNFR2 humanized mice (B-hTNFR2). Murine colon cancer MC38 cells (5×10⁵) were subcutaneously implanted into homozygous TNFR2 humanized mice (female, 8–9 weeks old, n = 6). Mice were grouped when tumor volume reached approximately 100 mm³, at which point they were treated with anti-human TNFR2 antibodies at the doses and schedules indicated in panel A. (B) Body weight changes during treatment. Anti-human TNFR2 antibody (in house) effectively controlled tumor growth in TNFR2 humanized mice (B-hTNFR2), demonstrating that TNFR2 humanized mice (B-hTNFR2) provide a powerful preclinical model for in vivo evaluation of anti-human TNFR2 antibodies. Values are expressed as mean ± SEM.
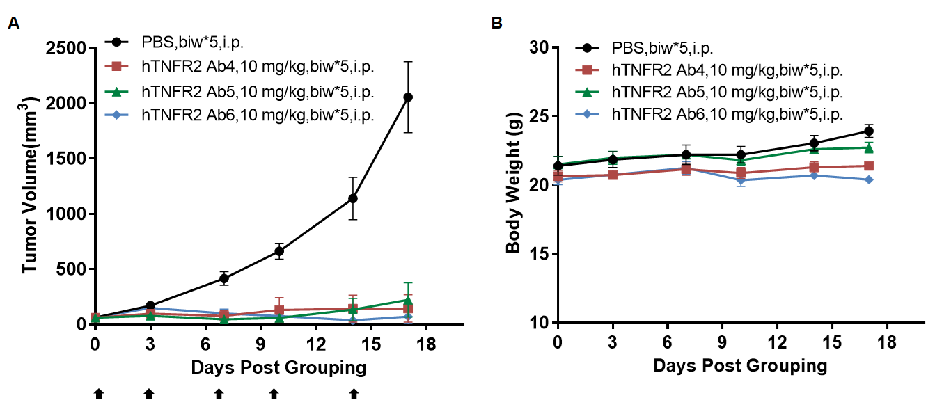
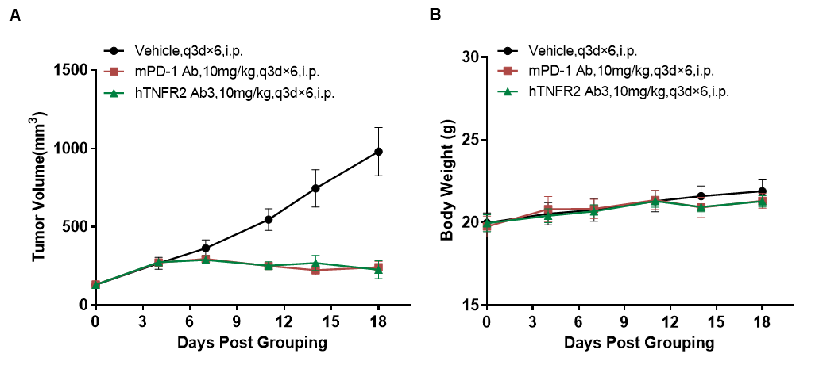
Antitumor activity of anti-human TNFR2 antibodies in TNFR2 humanized mice (B-hTNFR2). (A) Anti-human TNFR2 antibodies inhibited MC38 tumor growth in TNFR2 humanized mice (B-hTNFR2). Murine colon cancer MC38 cells (5×10⁵) were subcutaneously implanted into homozygous TNFR2 humanized mice (female, 5-8 week-old, n=4). Mice were grouped when tumor volume reached approximately 50 mm3, at which time they were treated with anti-human TNFR2 antibodies at the doses and schedules indicated in panel A. (B) Body weight changes during treatment. Anti-human TNFR2 antibodies effectively controlled tumor growth in TNFR2 humanized mice (B-hTNFR2), demonstrating that TNFR2 humanized mice (B-hTNFR2) provide a powerful preclinical model for in vivo evaluation of anti-human TNFR2 antibodies. Values are expressed as mean ± SEM. (All antibodies were provided by the clients.)
Q1: What are TNFR2 humanized mice?
B-hTNFR2 mice are genetically engineered models in which exons 2-6 of the mouse Tnfr2 gene, encoding the extracellular domain, are replaced by the corresponding human TNFR2 exons. This allows for the expression of the human TNFR2 protein in a mouse model.
Q2: Why are TNFR2 humanized mice important for immuno-oncology research?
TNFR2 is a key immunomodulator expressed on T cells and Tregs. This model enables the in vivo evaluation of anti-human TNFR2 therapeutics, which can inhibit tumor growth by modulating the tumor microenvironment, such as depleting Tregs and activating effector T cells.
Q3: How is human TNFR2 expression validated in these mice?
Human TNFR2 expression is exclusively detectable at both the mRNA and protein levels in homozygous B-hTNFR2 mice, as confirmed by RT-PCR, RT-qPCR, and flow cytometry using species-specific antibodies, while being absent in wild-type mice.
Q4: Does the humanization of TNFR2 affect normal immune development?
No. Comprehensive immune profiling demonstrates that the development, differentiation, and distribution of major immune cell populations (including T, B, and NK cells) in the spleen and lymph nodes of B-hTNFR2 mice are comparable to wild-type controls.
Q5: What is the evidence that this model is predictive for drug efficacy?
Multiple in vivo efficacy studies show that anti-human TNFR2 antibodies can dose-dependently inhibit tumor growth in B-hTNFR2 mice, validating it as a powerful preclinical model for evaluating anti-TNFR2 therapies.