| Strain Name |
C57BL/6N-Pdcd1tm3(PDCD1)Bcgen/Bcgen
|
Common Name | B-hPD-1 mice plus |
| Background | C57BL/6N | Catalog number | 110019 |
|
Related Genes |
CD279, PD-1, PD1, SLEB2, hPD-1, hPD-l, hSLE1 |
||
|
NCBI Gene ID |
18566 |
||
Protein expression analysis
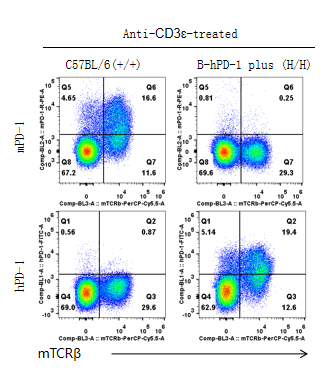
Strain specific PD-1 expression analysis in homozygous B-hPD-1 mice plus by flow cytometry. Splenocytes were collected from wild-type C57BL/6 mice (+/+) and homozygous B-hPD-1 mice plus (H/H) stimulated with anti-CD3ε in vivo, and analyzed by flow cytometry with species-specific anti-PD-1 antibodies. Mouse PD-1 was detectable in wild-type C57BL/6 mice. Human PD-1 was exclusively detectable in homozygous B-hPD-1 mice plus but not in wild-type mice.
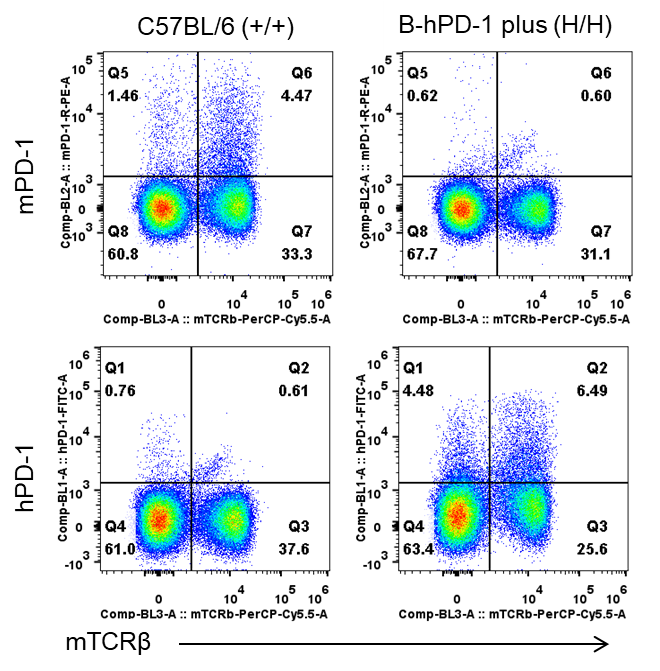
Strain specific PD-1 expression analysis in homozygous B-hPD-1 mice plus by flow cytometry. Splenocytes were collected from wild-type C57BL/6 mice (+/+) and homozygous B-hPD-1 mice plus (H/H) and analyzed by flow cytometry with species-specific anti-PD-1 antibodies. Mouse PD-1 was detectable in wild-type C57BL/6 mice. Human PD-1 was exclusively detectable in homozygous B-hPD-1 mice plus but not in wild-type mice.
Analysis of leukocytes cell subpopulation in spleen
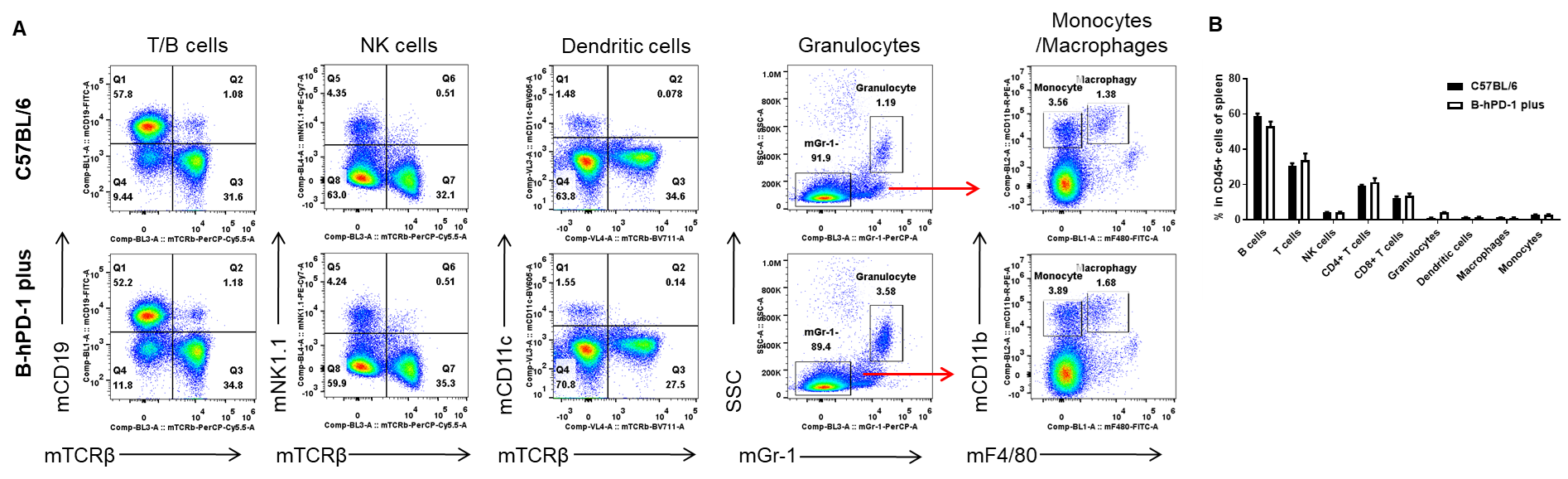
Analysis of spleen leukocyte subpopulations by FACS. Splenocytes were isolated from female C57BL/6 and B-hPD-1 mice plus (n=3, 7-week-old). Flow cytometry analysis of the splenocytes was performed to assess leukocyte subpopulations. A. Representative FACS plots. Single live cells were gated for the CD45+ population and used for further analysis as indicated here. B. Results of FACS analysis. Percent of T cells, B cells, NK cells, dendritic cells, granulocytes, monocytes and macrophages in homozygous B-hPD-1 mice plus were similar to those in the C57BL/6 mice, demonstrating that PD-1 humanized does not change the overall development, differentiation or distribution of these cell types in spleen. Values are expressed as mean ± SEM.
Analysis of T cell subpopulation in spleen
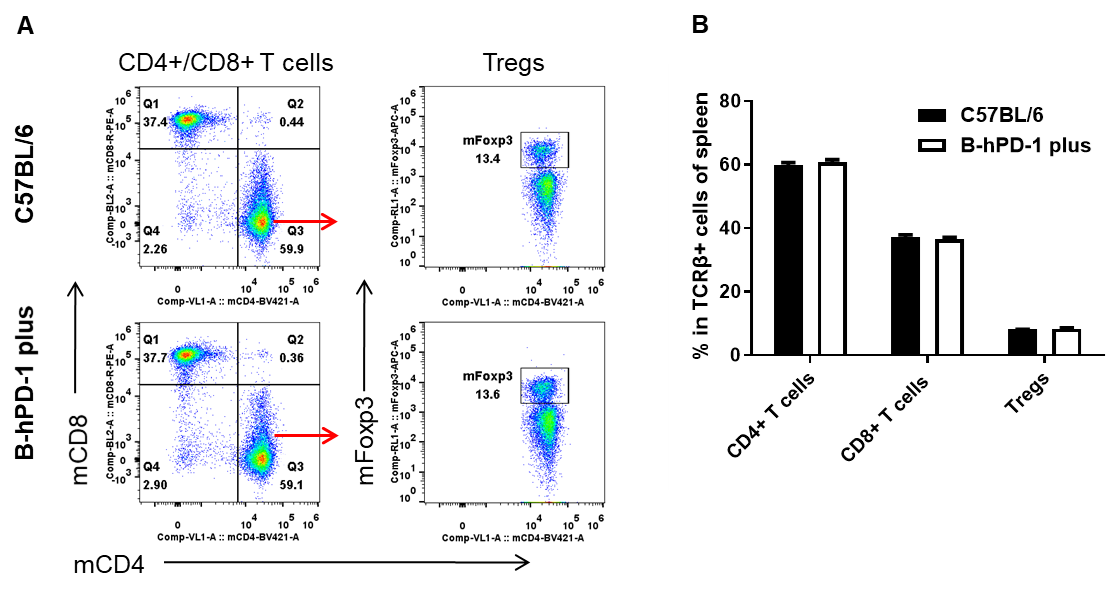
Analysis of spleen T cell subpopulations by FACS. Splenocytes were isolated from female C57BL/6 and B-hPD-1 mice plus (n=3, 7-week-old). Flow cytometry analysis of the splenocytes was performed to assess leukocyte subpopulations. A. Representative FACS plots. Single live CD45+ cells were gated for TCRβ+ T cell population and used for further analysis as indicated here. B. Results of FACS analysis. The percent of CD4+ T cells, CD8+ T cells and Tregs in homozygous B-hPD-1 mice plus were similar to those in the C57BL/6 mice, demonstrating that introduction of hPD-1 in place of its mouse counterpart does not change the overall development, differentiation or distribution of these T cell subtypes in spleen. Values are expressed as mean ± SEM.
Analysis of leukocytes cell subpopulation in lymph node
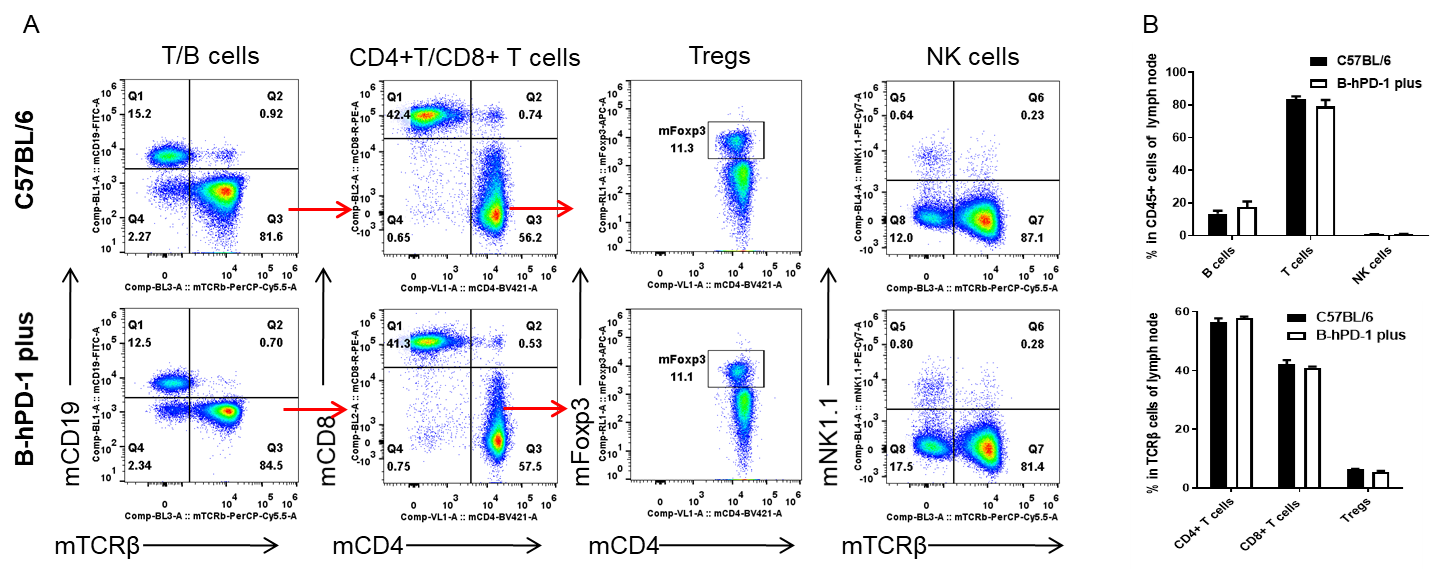
Analysis of lymph node leukocyte subpopulations by FACS. Lymph nodes were isolated from female C57BL/6 and B-hPD-1 mice plus (n=3, 7-week-old). Flow cytometry analysis of the leukocytes was performed to assess leukocyte subpopulations. A. Representative FACS plots. Single live cells were gated for the CD45+ population and used for further analysis as indicated here. B. Results of FACS analysis. Percent of T cells, B cells, CD4+ T cells, CD8+ T cells, Tregs and NK cells in homozygous B-hPD-1 mice plus were similar to those in the C57BL/6 mice, demonstrating that PD-1 humanized does not change the overall development, differentiation or distribution of these cell types in lymph node. Values are expressed as mean ± SEM.
T cells in B-hPD-1 plus mice bind anti-human PD-1 antibody
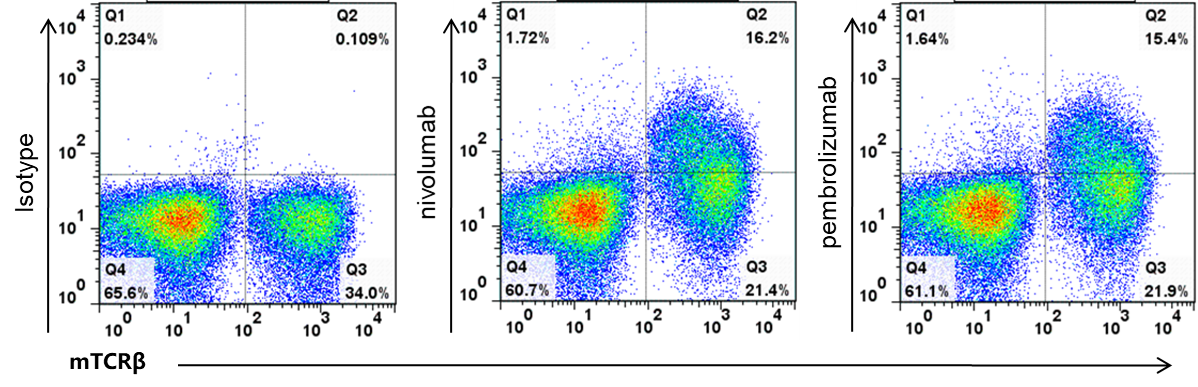
Analysis of splenocytes of B-hPD-1 mice plus by FACS. Splenocytes were isolated from female B-hPD-1 mice plus. Flow cytometry analysis of the splenocytes was performed to assess human PD-1 expression on T cells. Single live cells were gated for CD45+ population and used for further analysis as indicated here. Human PD-1 expression was detectable on T cells in B-hPD-1 mice plus as evidenced by nivolumab (in house) and pembrolizumab (in house) binding vs isotype control.
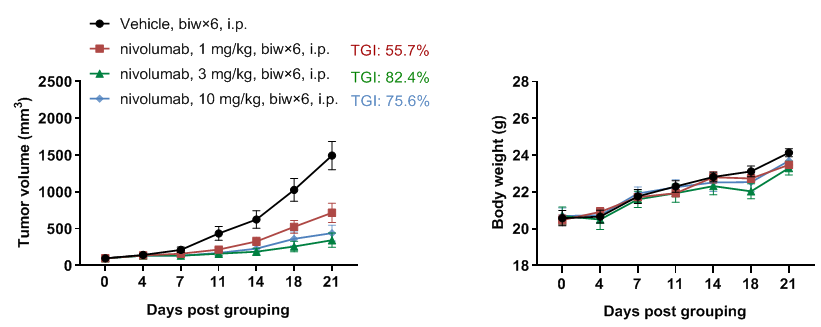
In vivo efficacy of anti-human PD-1 antibody
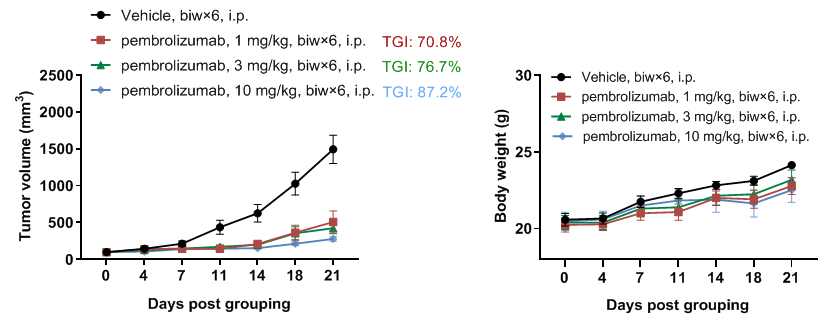
Antitumor activity of anti-human PD-1 antibody pembrolizumab (in house) in B-hPD-1 mice plus. (A) Anti-human PD-1 antibody inhibited MC38 tumor growth in B-hPD-1 mice plus. Murine colon cancer MC38 cells (5×105) were subcutaneously implanted into homozygous B-hPD-1 mice plus (female 7-week-old, n=5). Mice were grouped when tumor volume reached approximately 150 mm3, at which time they were treated with anti-human PD-1 antibody with doses and schedules indicated in panel. (B) Body weight changes during treatment. As shown in panel A, anti-human PD-1 antibody was efficacious in controlling tumor growth in B-hPD-1 mice plus, demonstrating that the B-hPD-1 mice plus provide a powerful preclinical model for in vivo evaluation of anti-human PD-1 antibodies. Values are expressed as mean ± SEM.
In vivo efficacy of anti-human PD-1 antibody