
C57BL/6N-B2mtm2(B2M/HLA-A2.1/H2-D)Bcgen/Bcgen • 110110

| Product name | B-HLA-A2.1 mice |
|---|---|
| Catalog number | 110110 |
| Strain name | C57BL/6N-B2mtm2(B2M/HLA-A2.1/H2-D)Bcgen/Bcgen |
| Strain background | C57BL/6N |
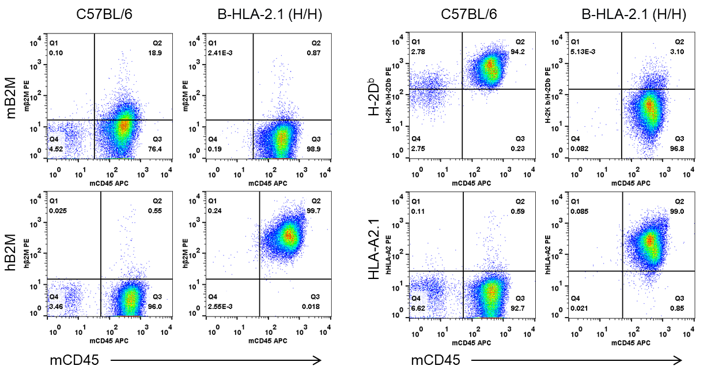
Strain specific B2M and HLA expression analysis in homozygous B-HLA-A2.1 mice by flow cytometry. Splenocytes from both wild-type C57BL/6 (+/+) and homozygous B-HLA-A2.1 mice (H/H) were analyzed by flow cytometry. Mouse B2M and H-2Db were detectable in the wild-type C57BL/6 mice. Human B2M and HLA-A2.1 were only detectable in the homozygous B-HLA-A2.1 mice.
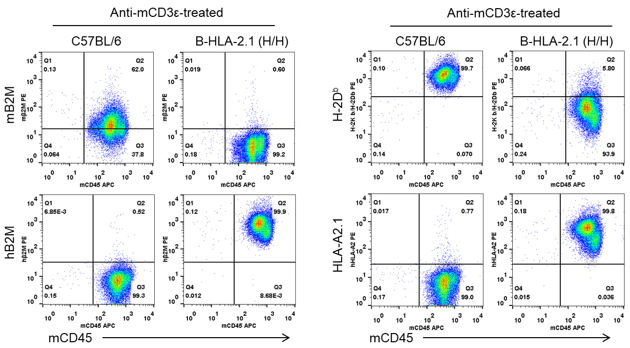
Strain specific B2M and HLA expression analysis in homozygous B-HLA-A2.1 mice by flow cytometry. Splenocytes from both wild-type C57BL/6 (+/+) and homozygous B-HLA-A2.1 mice (H/H) stimulated with anti-CD3ε in vivo, and analyzed by flow cytometry. Mouse B2M and H-2Db were detectable in the wild-type C57BL/6 mice. Human B2M and HLA-A2 were only detectable in the homozygous B-HLA-A2.1 mice.
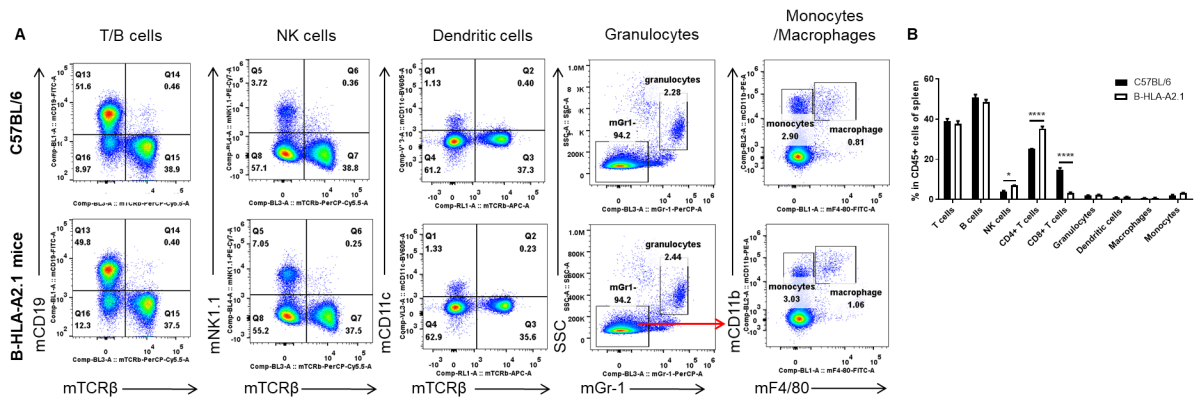
Analysis of spleen leukocyte subpopulations by FACS. Splenocytes were isolated from female C57BL/6 and B-HLA-A2.1 mice (n=3, 8-week-old). Flow cytometry analysis of the splenocytes was performed to assess leukocyte subpopulations. A. Representative FACS plots. Single live cells were gated for the CD45+ population and used for further analysis as indicated here. B. Results of FACS analysis. Percent of B cells, dendritic cells, granulocytes, monocytes and macrophages in homozygous B-HLA-A2.1 mice were similar to those in the C57BL/6 mice. Percent of CD8+ T cells were significantly decreased while percent of CD4+ T cells and NK cells were significantly increased, demonstrating that introduction of hB2M-HLA-A2.1-H-2D in place of mouse B2M may affected the development of CD8 + T cells, which in turn affected the proportion of T cell subtypes in the spleen. Values are expressed as mean ± SEM.
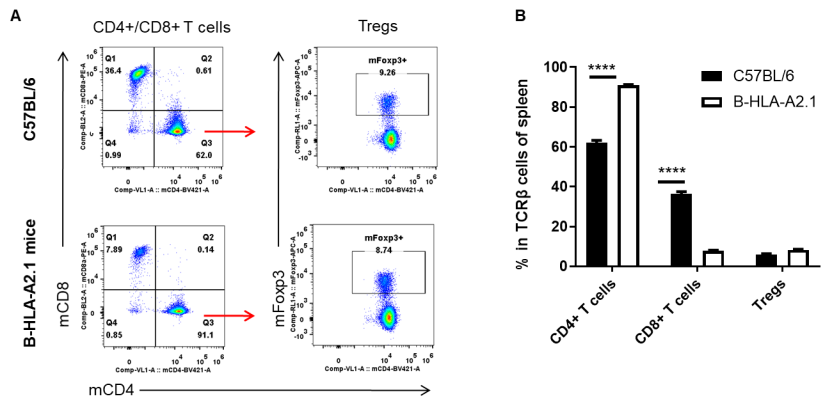
Analysis of spleen T cell subpopulations by FACS. Splenocytes were isolated from female C57BL/6 and B-HLA-A2.1 mice (n=3, 8-week-old). Flow cytometry analysis of the splenocytes was performed to assess leukocyte subpopulations. A. Representative FACS plots. Single live CD45+ cells were gated for TCRβ+ T cell population and used for further analysis as indicated here. B. Results of FACS analysis. The percent of Tregs in homozygous B-HLA-A2.1 mice were similar to those in the C57BL/6 mice. Percent of CD8+ T cells were significantly decreased while percent of CD4+ T cells were significantly increased, demonstrating that introduction of hB2M-HLA-A2.1-H-2D in place of mouse B2M may affected the development of CD8 + T cells, which in turn affected the proportion of T cell subtypes in the spleen. Values are expressed as mean ± SEM.
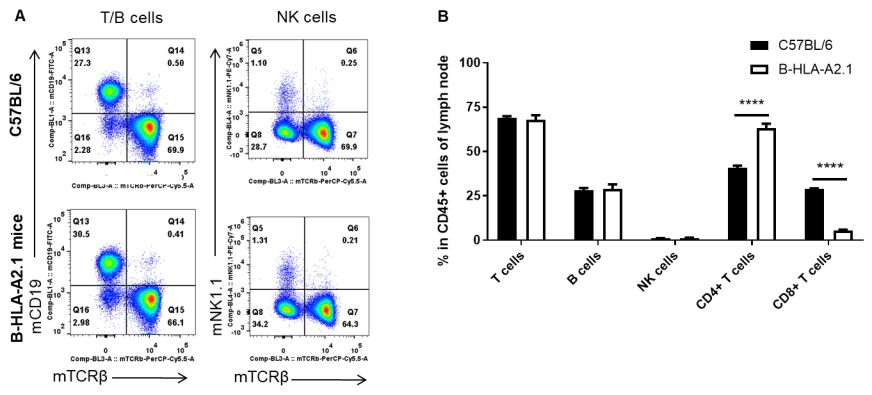
Analysis of lymph node leukocyte subpopulations by FACS. Lymph nodes were isolated from female C57BL/6 and B-HLA-A2.1 mice (n=3, 8-week-old). Flow cytometry analysis of the leukocytes was performed to assess leukocyte subpopulations. A. Representative FACS plots. Single live cells were gated for the CD45+ population and used for further analysis as indicated here. B. Results of FACS analysis. Percent of total T cells, B cells and NK cells in homozygous B-HLA-A2.1 mice were similar to those in the C57BL/6 mice. Percent of CD8+ T cells were significantly decreased while percent of CD4+ T cells were significantly increased, demonstrating that introduction of hB2M-HLA-A2.1-H-2D in place of mouse B2M may affected the development of CD8 + T cells, which in turn affected the proportion of T cell subtypes in lymph node. Values are expressed as mean ± SEM.
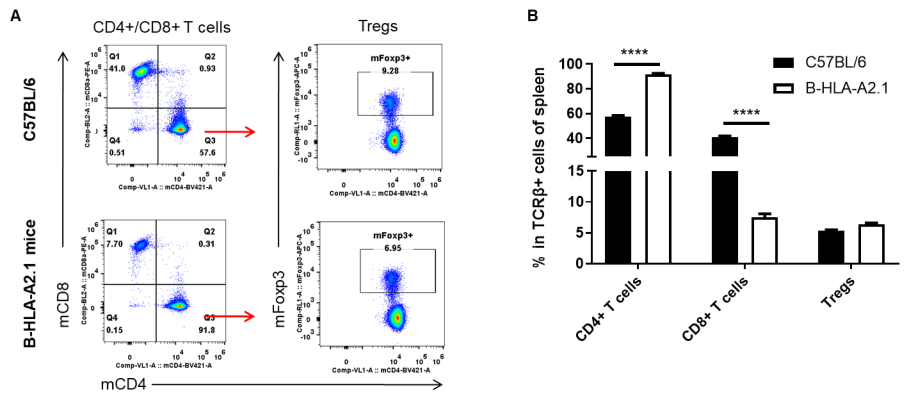
Analysis of lymph node T cell subpopulations by FACS. Leukocytes were isolated from lymph nodes of female C57BL/6 and B-HLA-A2.1 mice (n=3, 8-week-old). Flow cytometry analysis of the leukocytes was performed to assess leukocyte subpopulations. A. Representative FACS plots. Single live CD45+ cells were gated for TCRβ+ T cell population and used for further analysis as indicated here. B. Results of FACS analysis. The percent of Tregs in homozygous B-HLA-A2.1 mice were similar to those in the C57BL/6 mice. Percent of CD8+ T cells were significantly decreased while percent of CD4+ T cells were significantly increased, demonstrating that introduction of hB2M-HLA-A2.1-H-2D in place of mouse B2M may affected the development of CD8 + T cells, which in turn affected the proportion of T cell subtypes in lymph node. Values are expressed as mean ± SEM.
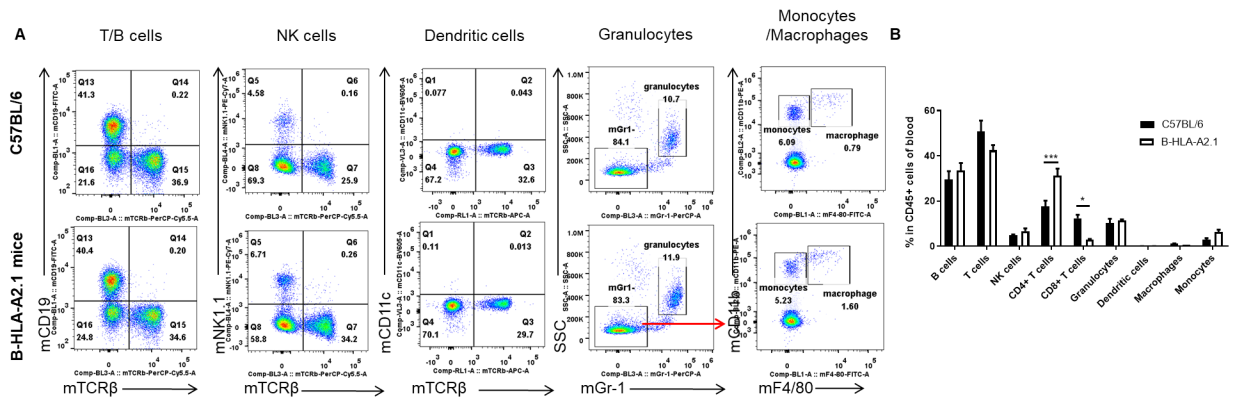
Analysis of blood leukocyte subpopulations by FACS. Blood cells were isolated from female C57BL/6 and B-HLA-A2.1 mice (n=3, 8-week-old). Flow cytometry analysis of the blood cells was performed to assess leukocyte subpopulations. A. Representative FACS plots. Single live cells were gated for the CD45+ population and used for further analysis as indicated here. B. Results of FACS analysis. Percent of total T cells, B cells, NK cells, dendritic cells, granulocytes, monocytes and macrophages in homozygous B-HLA-A2.1 mice were similar to those in the C57BL/6 mice. Percent of CD8+ T cells were significantly decreased while percent of CD4+ T cells were significantly increased, demonstrating that introduction of hB2M-HLA-A2.1-H-2D in place of mouse B2M may affected the development of CD8 + T cells, which in turn affected the proportion of T cell subtypes in blood. Values are expressed as mean ± SEM.
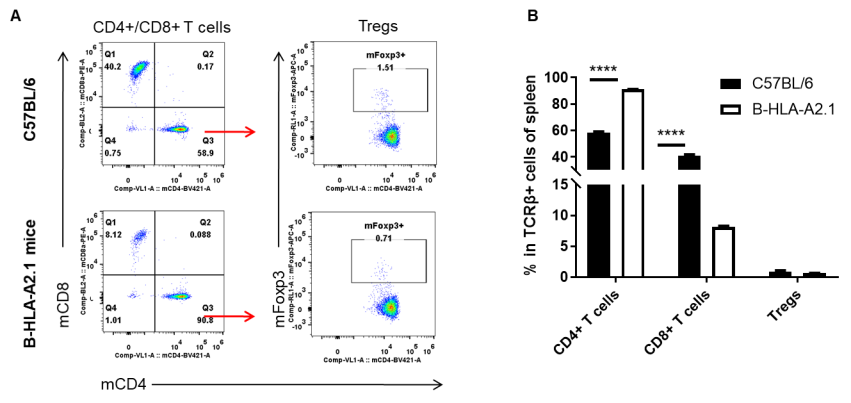
Analysis of blood T cell subpopulations by FACS. Blood cells were isolated from female C57BL/6 and B-HLA-A2.1 mice (n=3, 8-week-old). Flow cytometry analysis of the blood cells was performed to assess leukocyte subpopulations. A. Representative FACS plots. Single live CD45+ cells were gated for TCRβ+ T cell population and used for further analysis as indicated here. B. Results of FACS analysis. The percent of Tregs in homozygous B-HLA-A2.1 mice were similar to those in the C57BL/6 mice. Percent of CD8+ T cells were significantly decreased while percent of CD4+ T cells were significantly increased, demonstrating that introduction of hB2M-HLA-A2.1-H-2D in place of mouse B2M may affected the development of CD8 + T cells, which in turn affected the proportion of T cell subtypes in blood. Values are expressed as mean ± SEM.
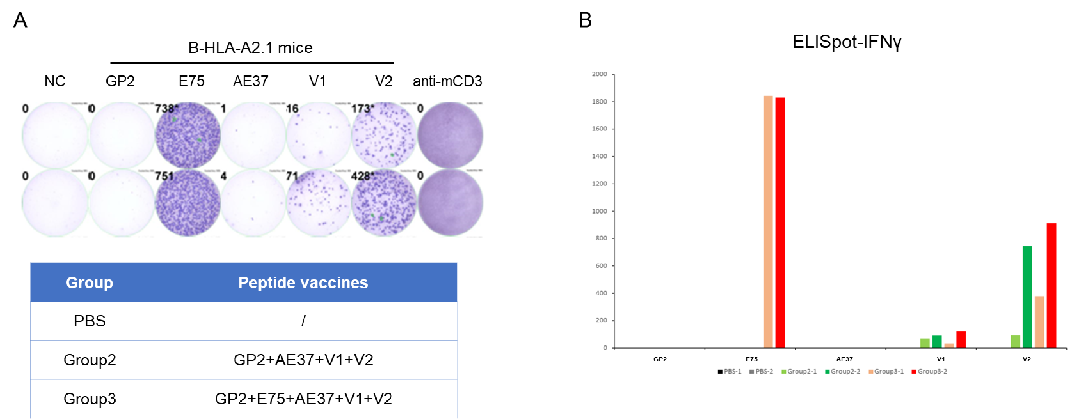
Detection of vaccine-induced immune responses in B-HLA-A2.1 mice by IFN-γ ELISpot assay. Female B-HLA-A2.1 mice at the age of 9–10 weeks were divided into PBS group, Group 2 and Group 3 (n=2), and then inoculated PBS or vaccines at the inside muscle of both legs. Three weeks after the last immunization, mice were sacrificed. The splenocytes were extracted, stimulated with individual peptide or target-unrelated polypeptide as negative control (NC) or anti-CD3 as positive control, and then measured for IFN-γ secretion. No significant difference in body weight among groups (Data was not shown). (A) Representative results showing stimulation of splenocytes harvested from immunized mice with negative control, or peptide vaccines, or positive control in duplicates. (B) Summary of results. The results demonstrate that B-HLA-2.1 mice provide a powerful preclinical model for in vivo evaluation of vaccines. NC: negative control. V1: the first peptide vaccine to be evaluated.
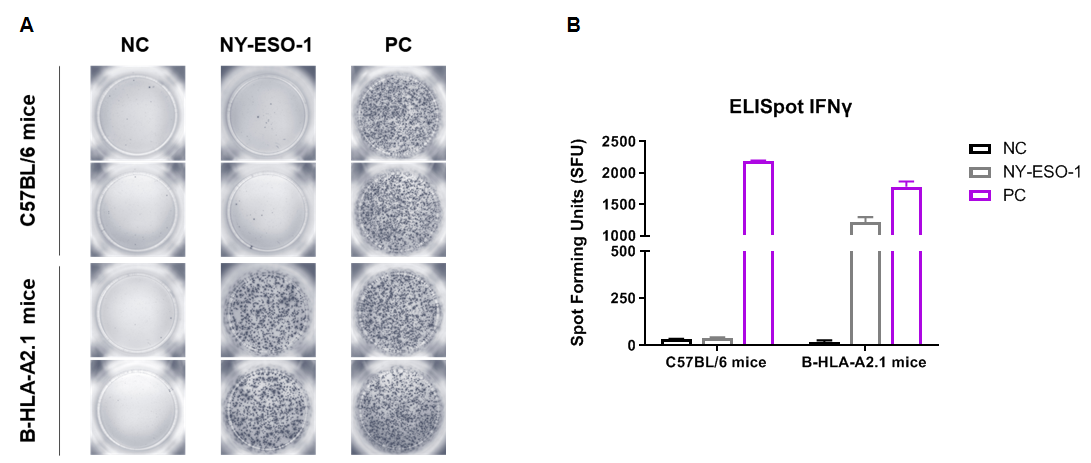
Detection of vaccine-induced immune responses in B-HLA-A2.1 mice by IFN-γ ELISpot assay. Male B-HLA-A2.1 mice at the age of 9–10 weeks were divided into PBS group and NY-ESO-1 group (n=3), and then inoculated PBS or vaccines at the inside muscle of both legs. One week after the last immunization, mice were sacrificed. The splenocytes were extracted, stimulated with individual peptide or target-unrelated polypeptide as negative control (NC) or PMA/Ionomycin as positive control, and then measured for IFN-γ secretion. No significant difference in body weight among groups (Data was not shown). (A) Representative results showing stimulation of splenocytes harvested from immunized mice with negative control, or peptide vaccines, or positive control in duplicates. (B) Summary of results. The results demonstrate that B-HLA-2.1 mice provide a powerful preclinical model for in vivo evaluation of vaccines. NC: negative control. PC: positive control.
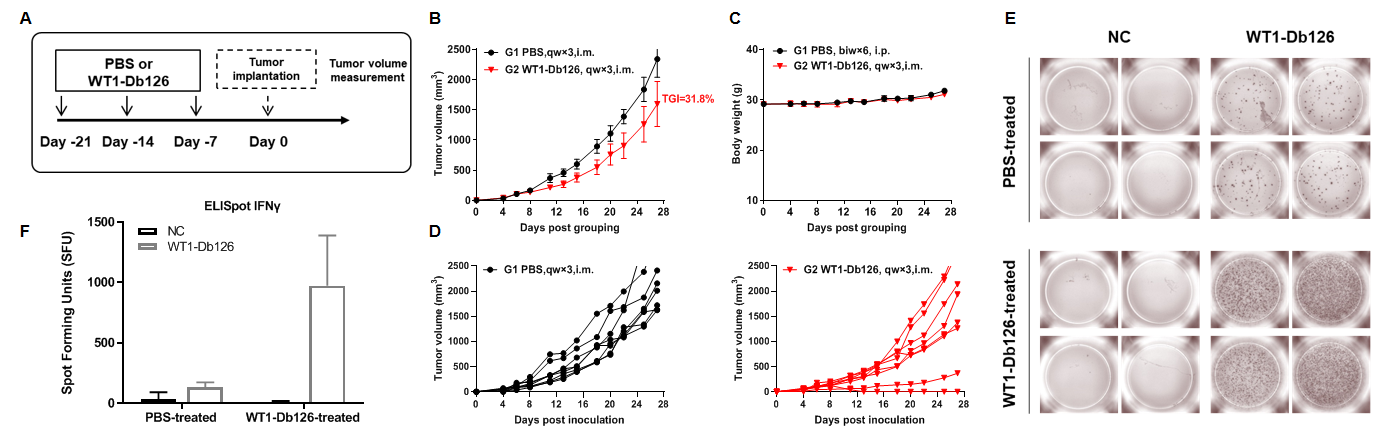
Antitumor activity of WT1-Db126 against syngeneic tumors. (A) Experimental scheme. (B) Antitumor activity of prophylactic treatment with WT1-Db126. B-HLA-A2.1 mice (n = 8/group) were vaccinated with WT1-Db126 (150 µg) or PBS. One week after the last immunization, B-HLA-A2.1/WT1 MC38 cells were inoculated into the right flank of the mice. (C) Body weight changes during treatment. As shown in panel A, WT1-Db126 peptides were efficacious in controlling tumor growth in B-HLA-A2.1 mice, demonstrating that the B-HLA-A2.1 mice provide a powerful preclinical model for in vivo evaluation of vaccines. Values are expressed as mean ± SEM. (D) B-HLA-A2.1/WT1 MC38 tumor cells growth of individual mice. (E) Representative results showing stimulation of splenocytes harvested from immunized mice with negative control, or peptide vaccines in duplicates. (F) Summary of results. These results demonstrate that B-HLA-2.1 mice provide a powerful preclinical model for in vivo evaluation of vaccines.
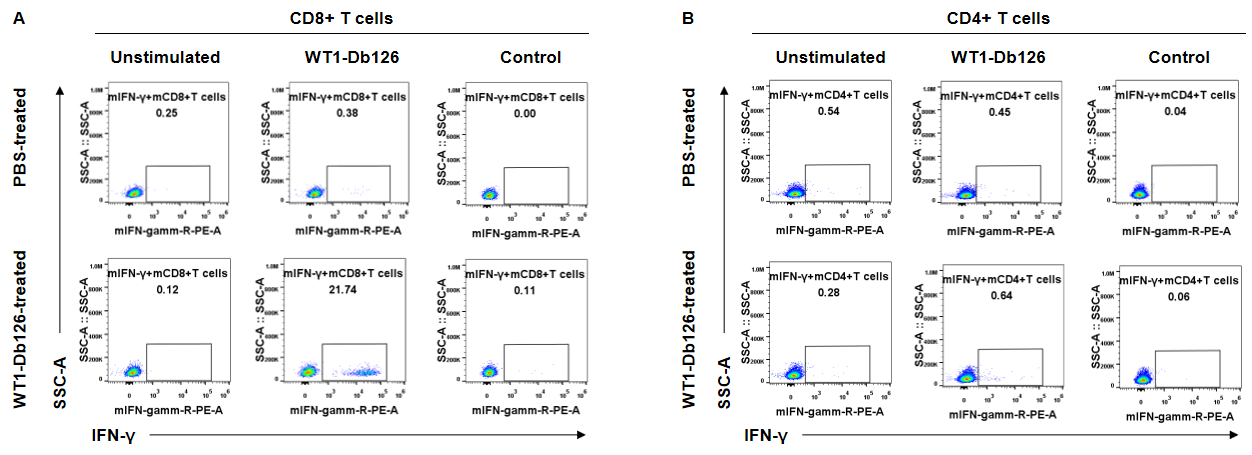
T-cell intracellular cytokine staining (ICS) assays. FACS plots demonstrating WT1-Db126-specific CD4+ and CD8+ T cells for B-HLA-A2.1 mice. CD8+ T cells produced primarily IFN-γ (A), whereas CD4+ cells didn’t produce IFN-γ (B). These results demonstrated that the IFN-γ was produced by the CD8+ T cells but not the CD4+ T cells.
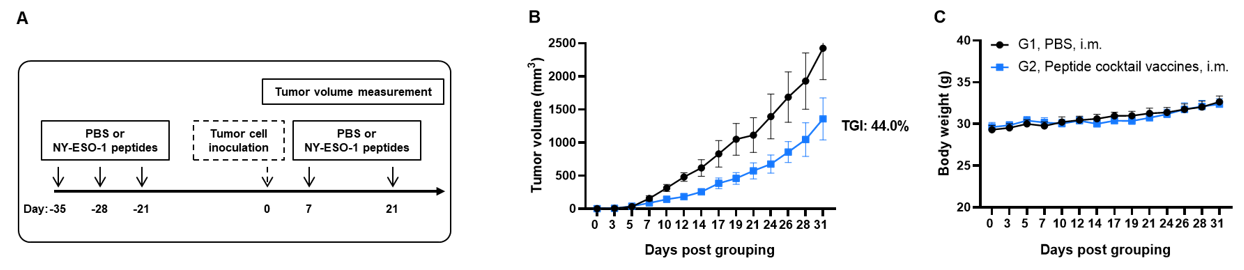
Antitumor activity of NY-ESO-1 peptides against syngeneic tumors. (A) Experimental scheme. (B) Antitumor activity of prophylactic treatment with NY-ESO-1 peptides. B-HLA-A2.1 mice (n=6 or 8/group) were vaccinated with PBS or NY-ESO-1 peptides(300 µg). Three weeks after the last immunization, B-HLA-A2.1/hNY-ESO-1 MC38 cells were inoculated into the right flank of the mice. (C) Body weight changes during treatment. As shown in panel B, NY-ESO-1 peptides were efficacious in controlling tumor growth in B-HLA-A2.1 mice, demonstrating that the B-HLA-A2.1 mice provide a powerful preclinical model for in vivo evaluation of vaccines. Values are expressed as mean ± SEM.
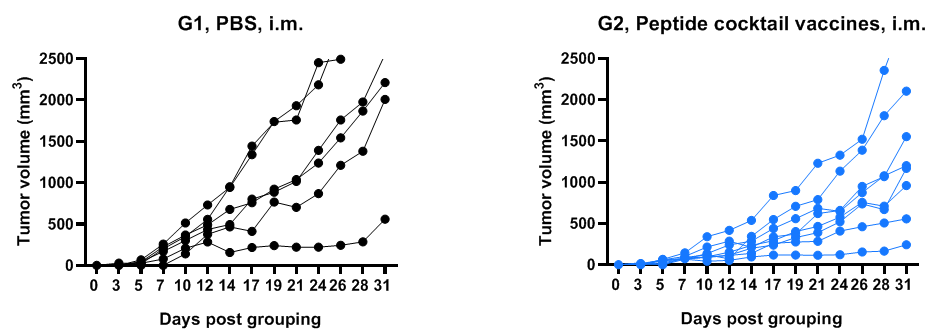
Antitumor activity of NY-ESO-1 peptides against syngeneic tumors. B-HLA-A2.1/hNY-ESO-1 MC38 tumor cells growth of individual mouse.
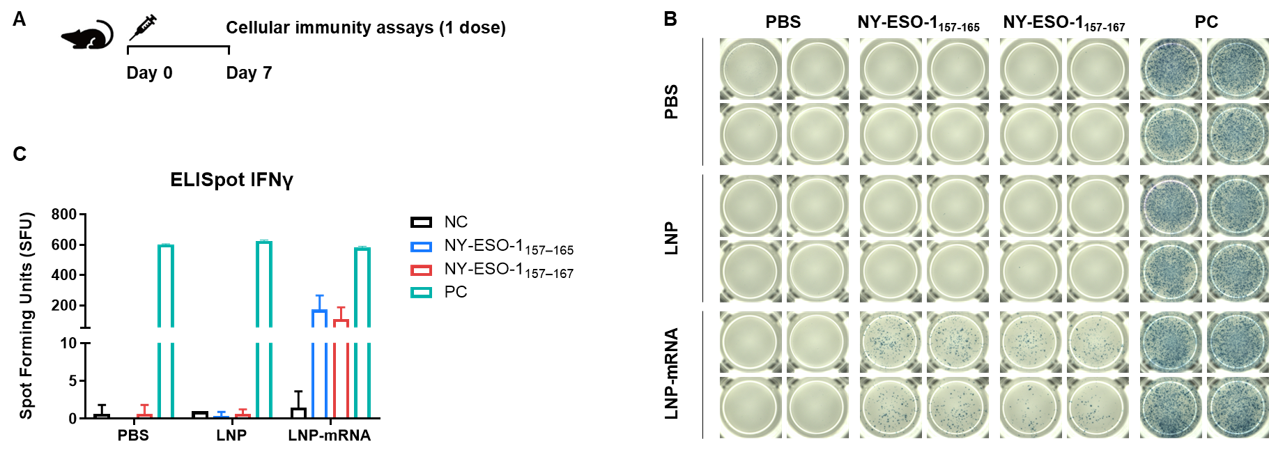
Detection of vaccine-induced immune responses in B-HLA-A2.1 mice by IFN-γ ELISpot assay. (A) Scheme of vaccination and testing. Male B-HLA-A2.1 mice at the age of 9–10 weeks were divided into PBS group, LNP group and LNP-mRNA group (n = 3), and then inoculated PBS, LNP or LNP-mRNA at the inside muscle of both legs. Mice were vaccinated with PBS, LNP or LNP-mRNA one time. One week after the immunization, mice were sacrificed. The splenocytes were extracted, stimulated with individual peptide, or no peptide as negative control (NC), or PMA/Ionomycin as positive control, and then measured for IFN-γ secretion. No significant difference in body weight among groups (Data was not shown). (B) Representative results showing stimulation of splenocytes harvested from immunized mice with negative control, or peptide vaccines, or positive control in duplicates. (C) Summary of results. The results demonstrate that B-HLA-2.1 mice provide a powerful preclinical model for in vivo evaluation of vaccines. NC: negative control. PC: positive control.
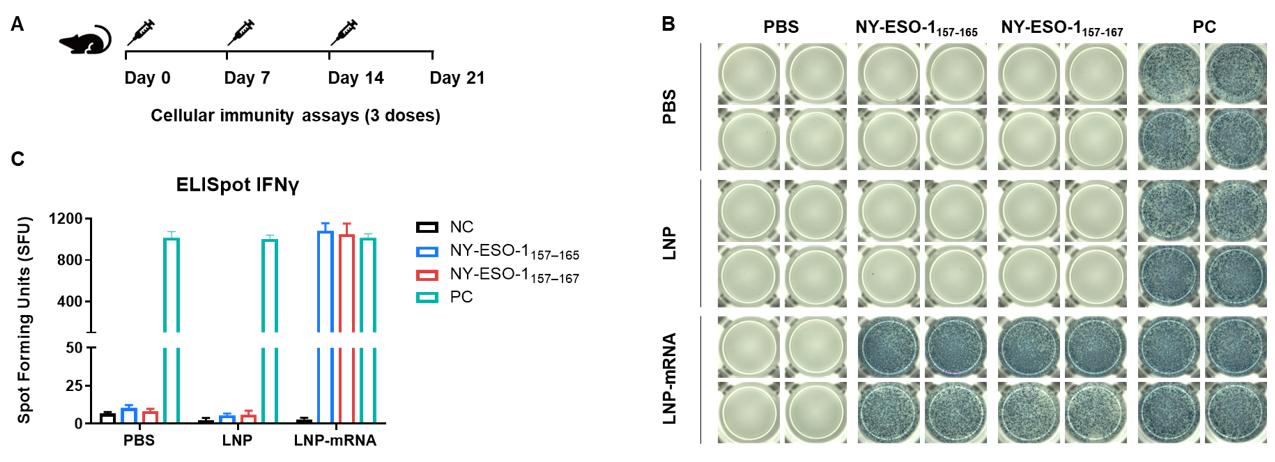
Detection of vaccine-induced immune responses in B-HLA-A2.1 mice by IFN-γ ELISpot assay. (A) Scheme of vaccination and testing. Male B-HLA-A2.1 mice at the age of 9–10 weeks were divided into PBS group, LNP group and LNP-mRNA group (n=3), and then inoculated PBS, LNP or LNP-mRNA at the inside muscle of both legs. Mice were vaccinated with PBS, LNP or LNP-mRNA three times at 1-week interval. One week after the last immunization, mice were sacrificed. The splenocytes were extracted, stimulated with individual peptide, or no peptide as negative control (NC), or PMA/Ionomycin as positive control, and then measured for IFN-γ secretion. No significant difference in body weight among groups (Data was not shown). (B) Representative results showing stimulation of splenocytes harvested from immunized mice with negative control, or peptide vaccines, or positive control in duplicates. (C) Summary of results. The results demonstrate that B-HLA-2.1 mice provide a powerful preclinical model for in vivo evaluation of vaccines. NC: negative control. PC: positive control.
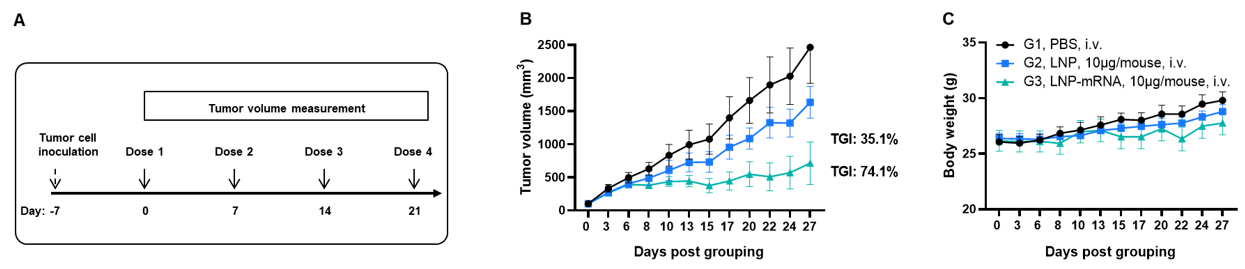
Antitumor activity of NY-ESO-1 mRNA vaccine against syngeneic tumors. (A) Experimental scheme. (B) Antitumor activity of therapeutic treatment with mRNA vaccine. B-HLA-A2.1 mice (n = 6/group) were vaccinated with PBS, LNP or LNP-mRNA. B-HLA-A2.1 mice were implanted subcutaneously with 5 × 105 B-HLA-A2.1/hNY-ESO-1 MC38 tumor cells in the right flank and immunized four times at 1-week intervals with PBS, LNP or mRNA vaccines via intravenous (i.v.) injection on days 0, 7, 14 and 21 after tumor volume reached approximately 100 mm3. As shown in panel B, the mRNA vaccines demonstrated robust inhibition of tumor growth and significantly prolonged overall survival compared to the control group. C. Body weight changes during tumor growth observation. These results demonstrate that B-HLA-2.1 mice provide a powerful preclinical model for in vivo evaluation of mRNA vaccines. Values are expressed as mean ± SEM.
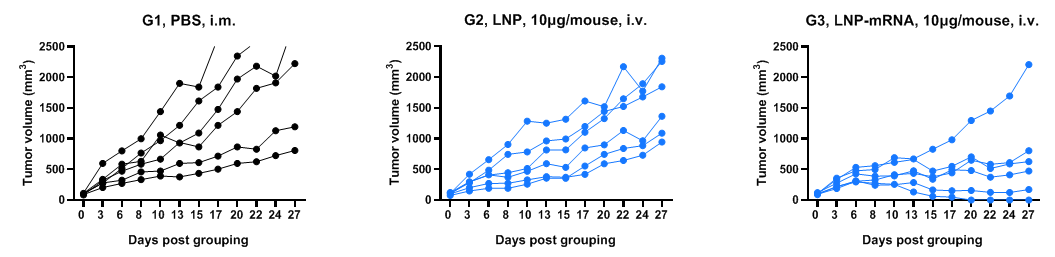
Antitumor activity of mRNA against syngeneic tumors. B-HLA-A2.1/hNY-ESO-1 MC38 tumor cells growth of individual mouse.
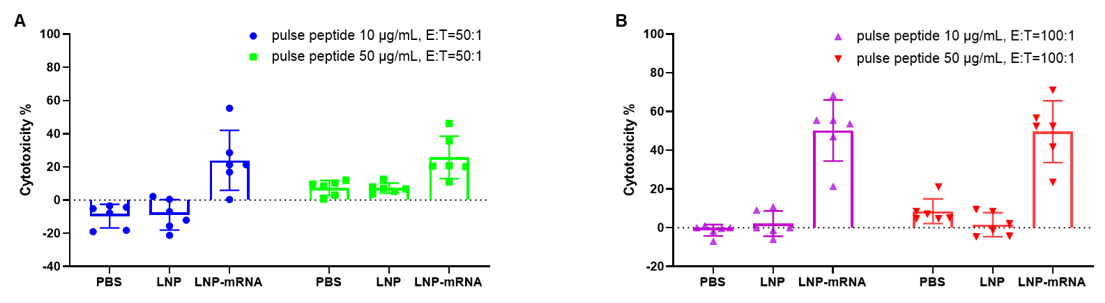
LDH release assay for cytotoxicity of CTLs from B-HLA-A2.1 mice immunized with PBS, LNP or LNP-mRNA against the B-HLA-A2.1/hNY-ESO-1 MC38 cell line. Cytotoxic activities of CTLs isolated from the splenocytes of immunized mice against NY-ESO-1 peptides pulsed B-HLA-A2.1/hNY-ESO-1 MC38 cell line were detected by LDH release assay at effector-to-target ratio of 50:1 or 100:1 with two different peptide concentrations, 10μg or 50μg.

Vaccination LNP-mRNA generates specific effector CD8+ T cells in spleens. Spleens from B-HLA-A2.1/hNY-ESO-1 MC38 tumor-bearing mice that were immunized with the PBS, empty LNP or LNP-mRNA were analyzed on day 27. Analysis of CD8+T, CD4+ T and Treg cells in the spleens determined by the flow cytometric assay. For spleen T cells(A) and CD8+T cells(B), the percentage(in CD45+ cells) were significantly elevated. LNP-mRNA generated frequencies of tetramer+ CD8+ T cells at approximately 50% of total CD8+ T cells in spleens(E). The CD8+ T cells had an obviously lower frequency in the naïve(F) and central memory(G) and were mainly localized in the effector memory(H). The IFNγ were mainly secreted by CD8+ T cells(I) but not CD4+ T cells(J).
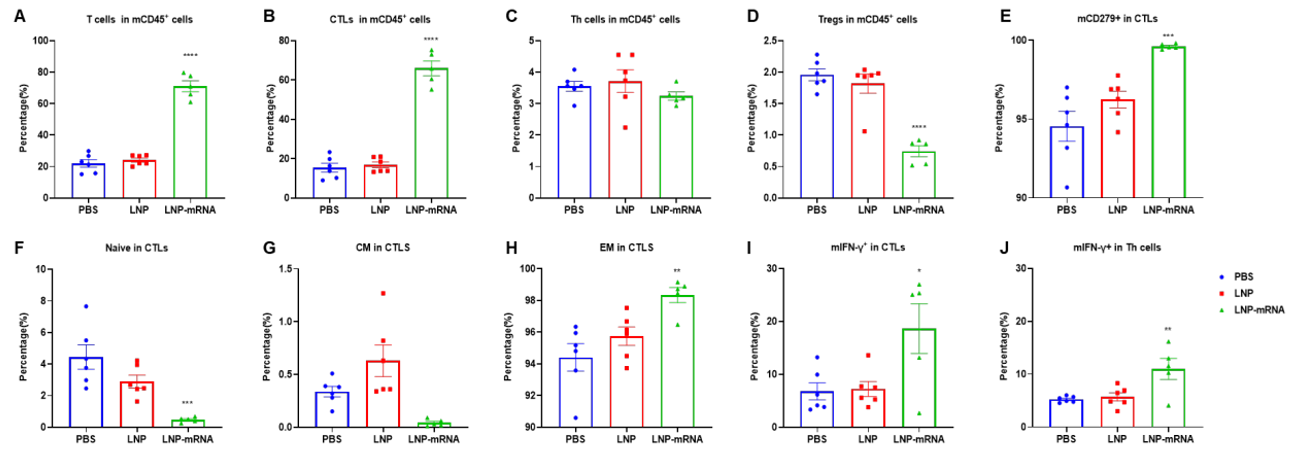
LNP-mRNA enhances beneficial repertoire of anti-tumor T cells. Tumors from B-HLA-A2.1/hNY-ESO-1 MC38 tumor-bearing mice that were immunized with the PBS, empty LNP or LNP-mRNA were analyzed on day 27. Analysis of CD8+T, CD4+ T and Treg cells in the tumors determined by the flow cytometric assay. For tumor-infiltrated T(A) and CD8+T cells(B), the percentage(in CD45+ cells) were significantly elevated. In contrast, the frequencies of Tregs was significantly diminished in LNP-mRNA group(D). Tumor-infiltrated CD8+ T cells had an obviously lower frequency in the naïve(F) and the effector memory(EM) CD8+T cells was increased significantly(H). The IFNγ in Both CD8+ T cells(I) and CD4+T cells(J) produced IFNγ.
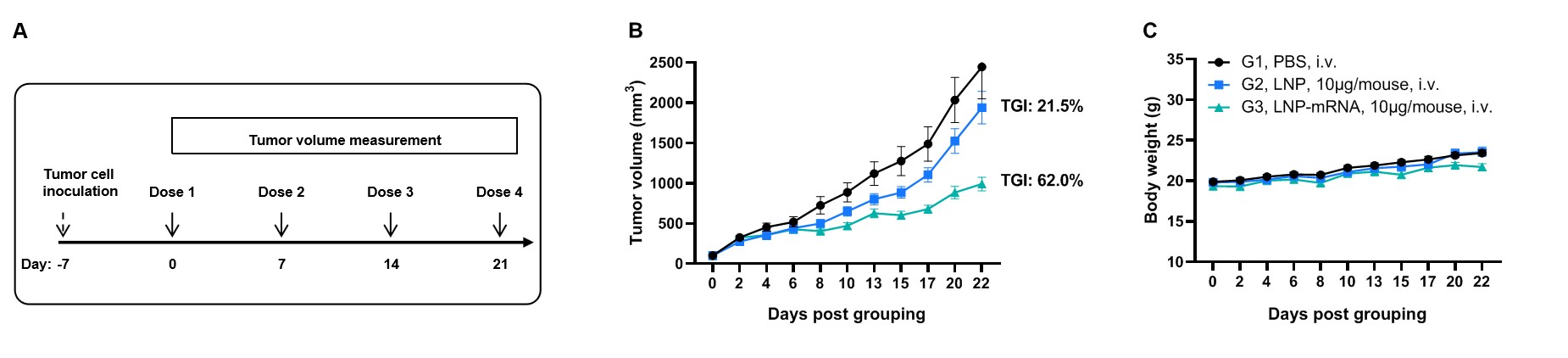
Antitumor activity of GP100 mRNA vaccine against syngeneic tumors. (A) Experimental scheme. (B) Antitumor activity of therapeutic treatment with mRNA vaccine. B-HLA-A2.1 mice (n = 8/group) were vaccinated with PBS, LNP or LNP-mRNA. B-HLA-A2.1 mice were implanted subcutaneously with 1×106 B-HLA-A2.1/hGP100 MC38 tumor cells in the right flank and immunized four times at 1-week intervals with PBS, LNP or mRNA vaccines via intravenous (i.v.) injection on days 0, 7, 14 and 21 after tumor volume reached approximately 100 mm3. As shown in panel B, the mRNA vaccines demonstrated robust inhibition of tumor growth and significantly prolonged overall survival compared to the control group. C. Body weight changes during tumor growth observation. These results demonstrate that B-HLA-2.1 mice provide a powerful preclinical model for in vivo evaluation of mRNA vaccines. Values are expressed as mean ± SEM.
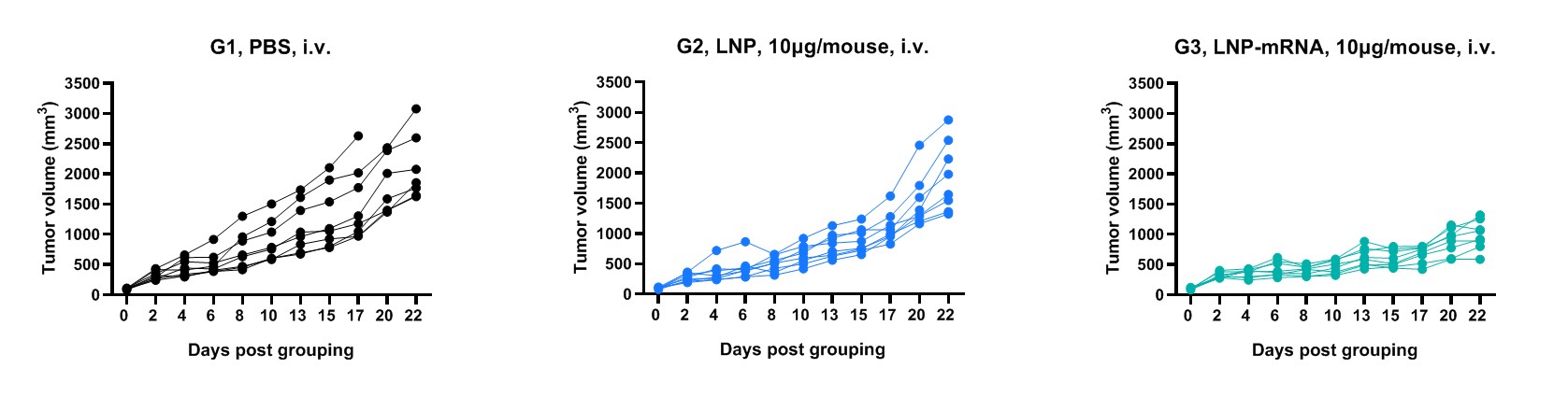
Antitumor activity of mRNA against syngeneic tumors. B-HLA-A2.1/hGP100 MC38 tumor cells growth of individual mice.
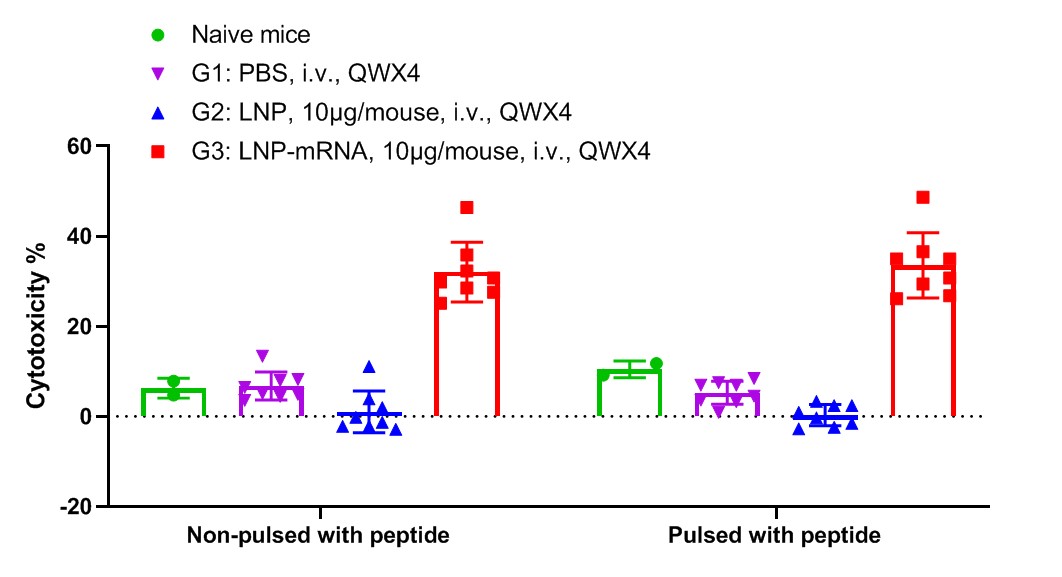
LDH release assay for cytotoxicity of CTLs from B-HLA-A2.1 mice immunized with PBS, LNP or LNP-mRNA against the B-HLA-A2.1/hGP100 MC38 cell line. Spleens from B-HLA-A2.1/hGP100 MC38 tumor-bearing mice that were immunized with the PBS, empty LNP or LNP-mRNA were collected on day 22. Cytotoxic activities of the splenocytes of immunized mice against GP100 peptides pulsed B-HLA-A2.1/hGP100 MC38 cell line were detected by LDH release assay at effector-to-target ratio of 100:1 with or without 10μg peptide.
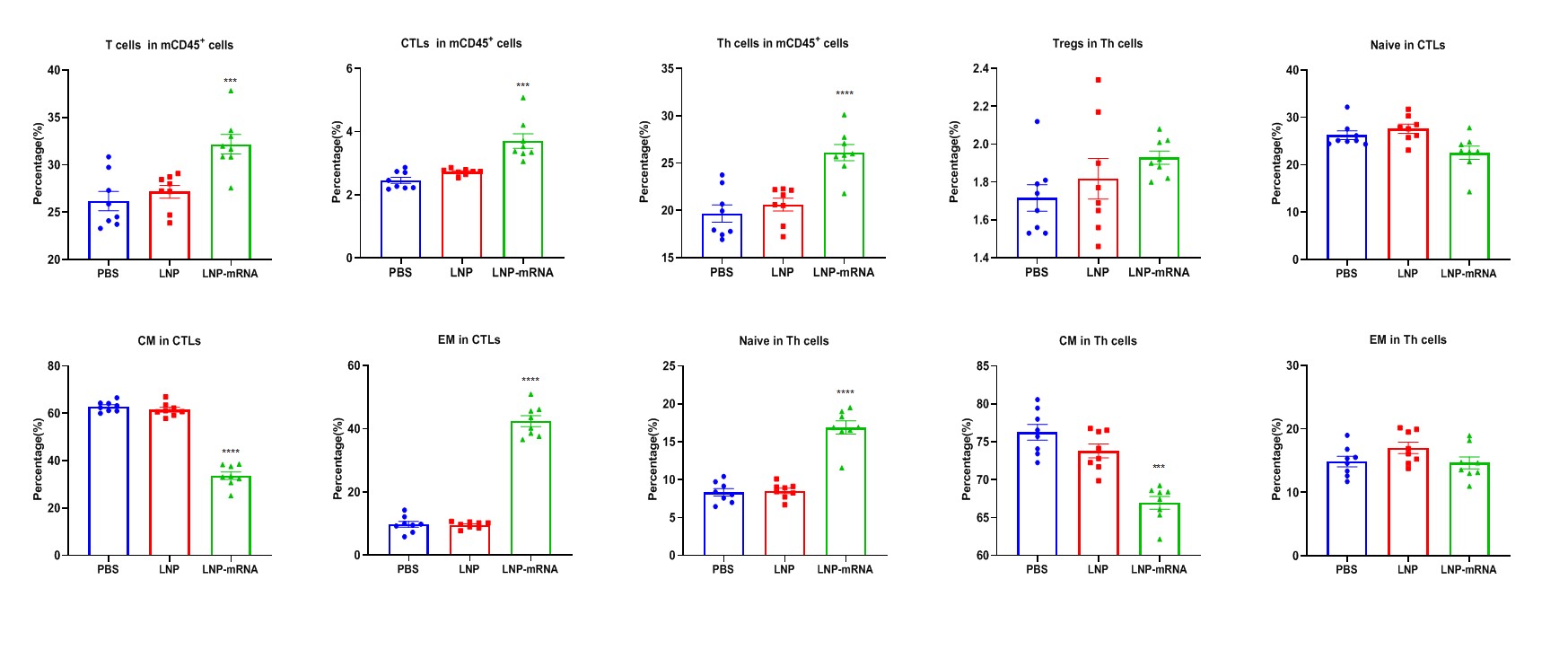
Vaccination LNP-mRNA generates specific effector CD8+ T cells in spleens. Spleens from B-HLA-A2.1/hGP100 MC38 tumor-bearing mice that were immunized with the PBS, empty LNP or LNP-mRNA were analyzed on day 22. Analysis of CD8+ T, CD4+ T and Treg cells in the spleens determined by the flow cytometric assay. For spleen T cells(A), and CD8+ T cells(B), the percentages (in CD45+ cells) were significantly elevated. The CD8+ T cells had an obviously lower frequency in the central memory(F) and were mainly localized in the effector memory(G), while the trend of naïve Th cells was opposite to that of CD8+T cells(H).
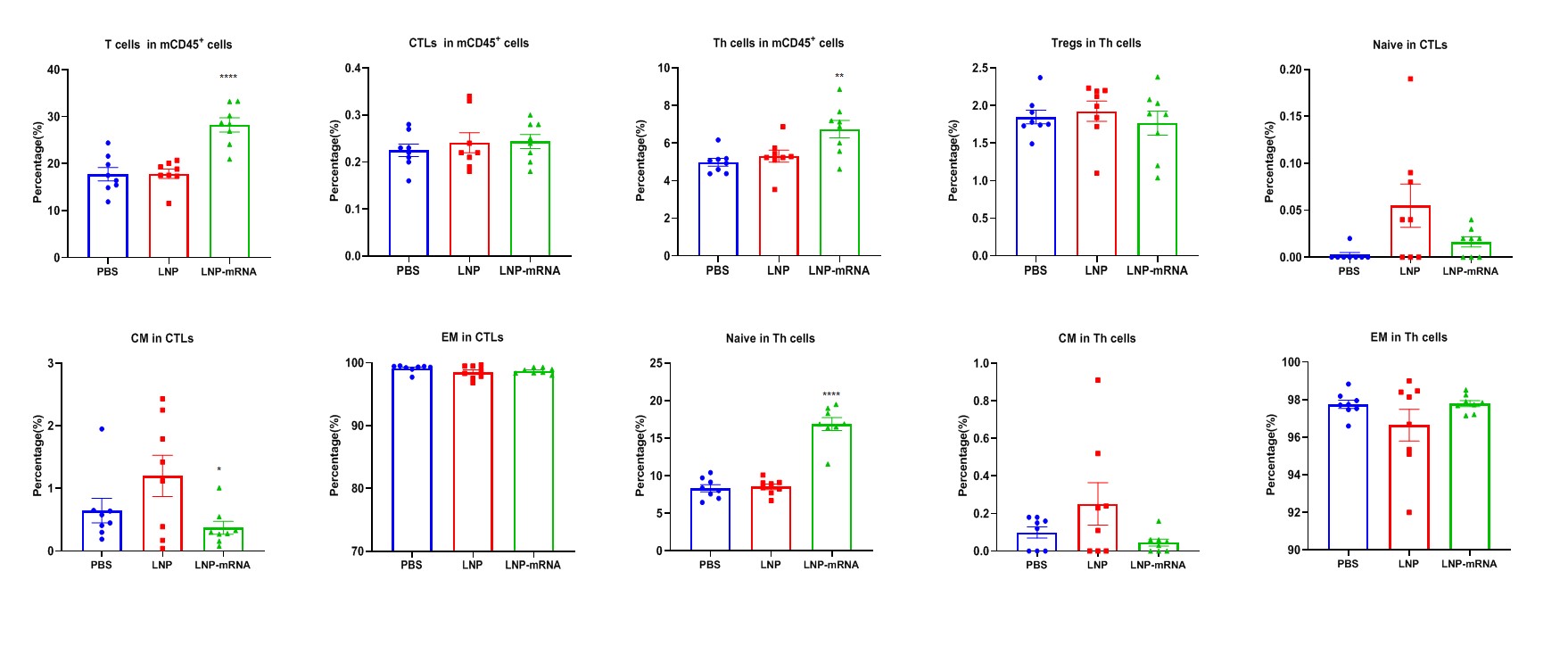
LNP-mRNA enhances beneficial repertoire of anti-tumor T cells. Tumors from B-HLA-A2.1/hGP100 MC38 tumor-bearing mice that were immunized with the PBS, empty LNP or LNP-mRNA were analyzed on day 22. Analysis of CD8+ T, CD4+ T and Treg cells in the tumors determined by the flow cytometric assay. For tumor-infiltrated T(A) and CD4+ T cells(C), the percentages(in CD45+ cells) were significantly elevated. In contrast, the frequencies of CD8+ T(B) and Tregs(D) were not significantly changed in LNP-mRNA group. Tumor-infiltrated naïve CD4+T cells was increased significantly(H).
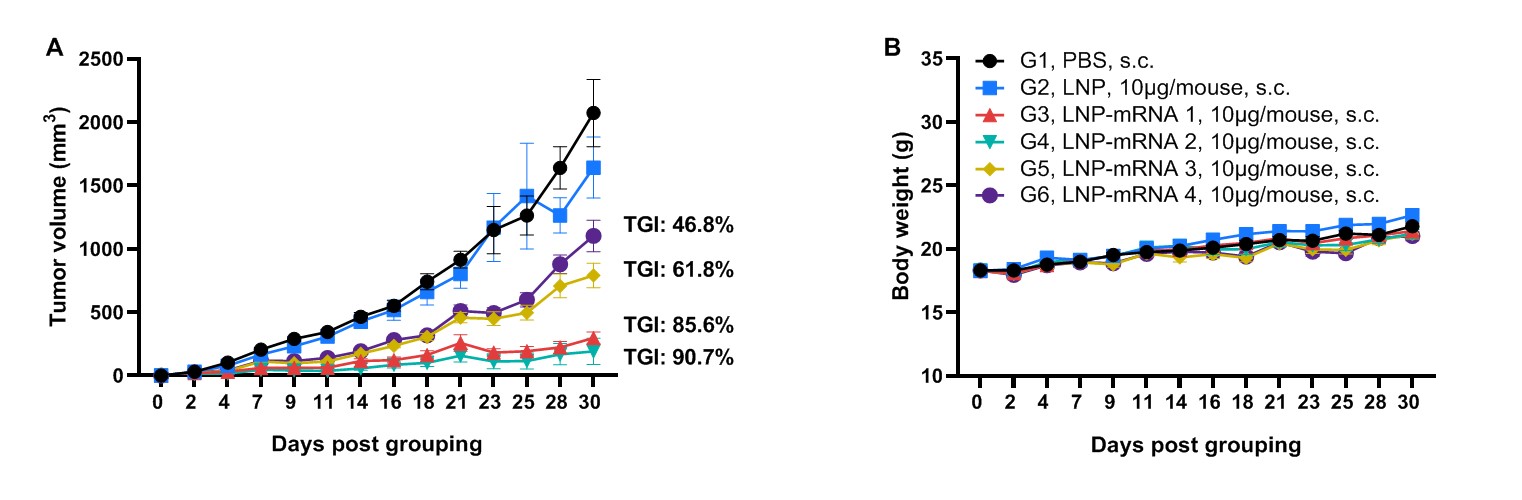
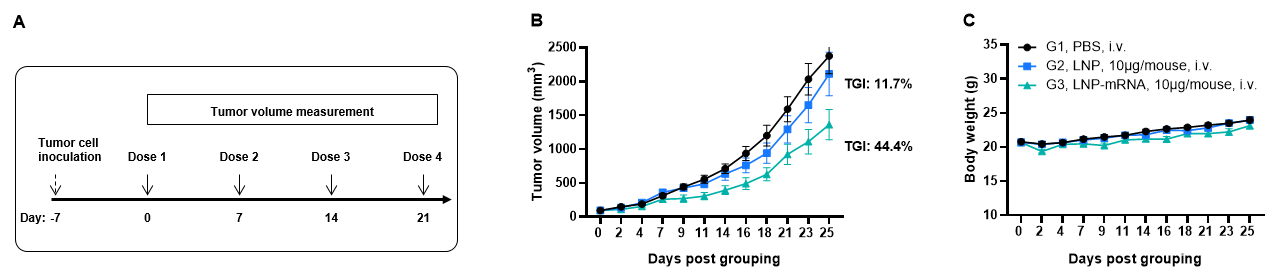
Antitumor activity of MAGEA3 mRNA vaccine against syngeneic tumors. (A) Antitumor activity of therapeutic treatment with mRNA vaccine. B-HLA-A2.1 mice (n = 8/group) were vaccinated with PBS, LNP or different LNP-mRNAs (provided by the client). B-HLA-A2.1 mice were implanted subcutaneously with 1×106 B-HLA-A2.1/hMAGEA3 MC38 tumor cells in the right flank and immunized with PBS, LNP or mRNA vaccines via intravenous (s.c.) injection. As shown in panel A, the mRNA vaccines demonstrated robust inhibition of tumor growth and significantly prolonged overall survival compared to the control group. B. Body weight changes during tumor growth observation. These results demonstrate that B-HLA-2.1 mice provide a powerful preclinical model for in vivo evaluation of mRNA vaccines. Values are expressed as mean ± SEM.
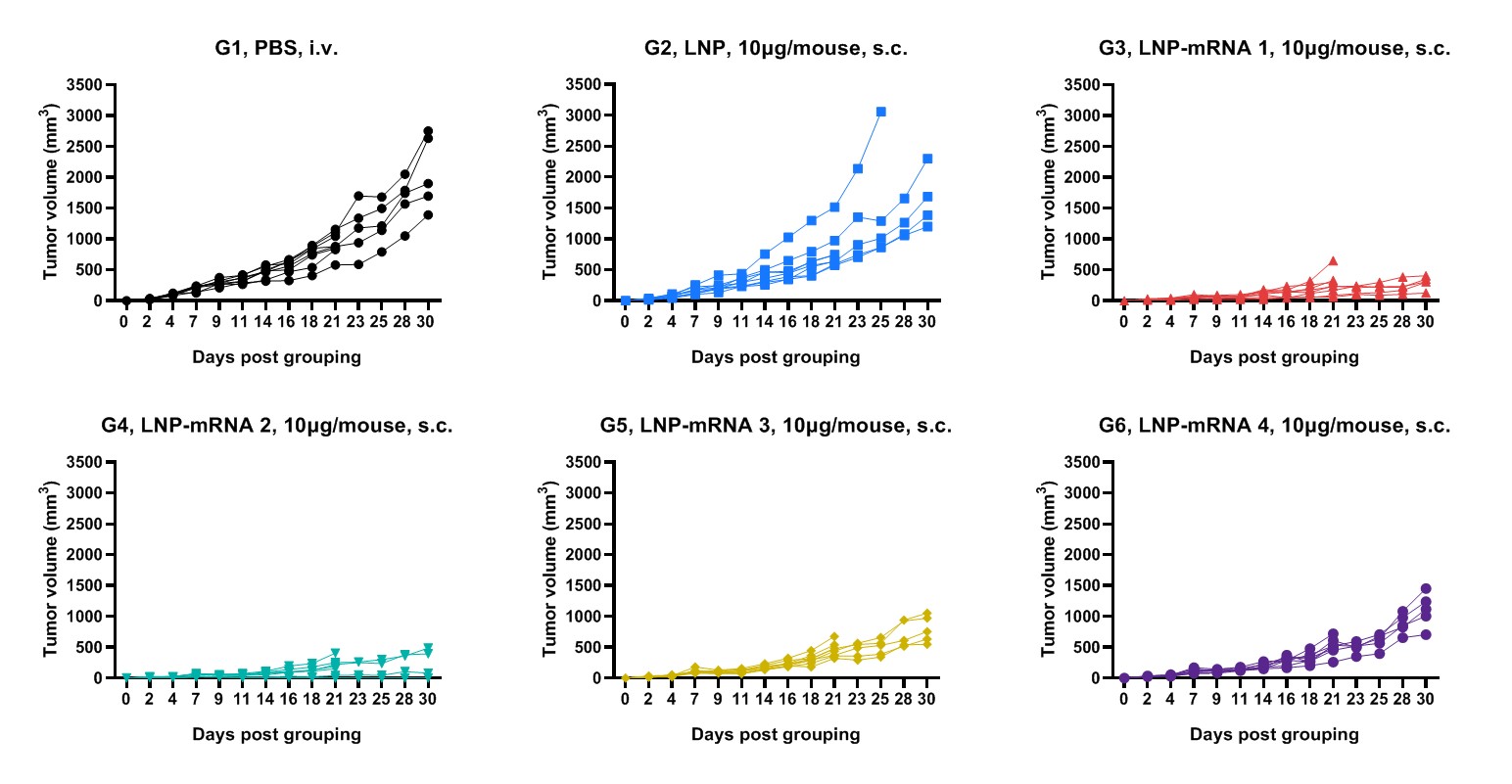
Antitumor activity of mRNA against syngeneic tumors. B-HLA-A2.1/hMAGEA3 MC38 tumor cells growth of individual mice.
Antitumor activity of AFP mRNA vaccine against syngeneic tumors. (A) Experimental scheme. (B) Antitumor activity of therapeutic treatment with mRNA vaccine. B-HLA-A2.1 mice (n = 8/group) were vaccinated with PBS, LNP or LNP-mRNA. B-HLA-A2.1 mice were implanted subcutaneously with 1×106 B-HLA-A2.1/hAFP MC38 tumor cells in the right flank and immunized four times at 1-week intervals with PBS, LNP or mRNA vaccines via intravenous (i.v.) injection on days 0, 7, 14 and 21 after tumor volume reached approximately 100 mm3. As shown in panel B, the mRNA vaccines demonstrated robust inhibition of tumor growth and significantly prolonged overall survival compared to the control group. C. Body weight changes during tumor growth observation. These results demonstrate that B-HLA-2.1 mice provide a powerful preclinical model for in vivo evaluation of mRNA vaccines. Values are expressed as mean ± SEM.
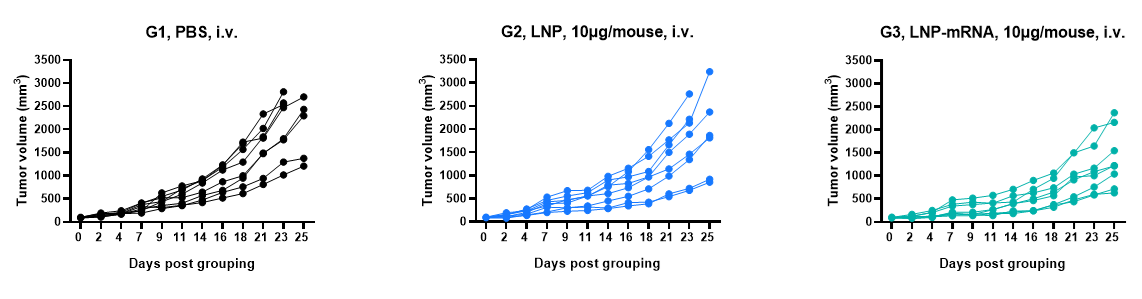
Antitumor activity of mRNA against syngeneic tumors. B-HLA-A2.1/hAFP MC38 tumor cells growth of individual mice.
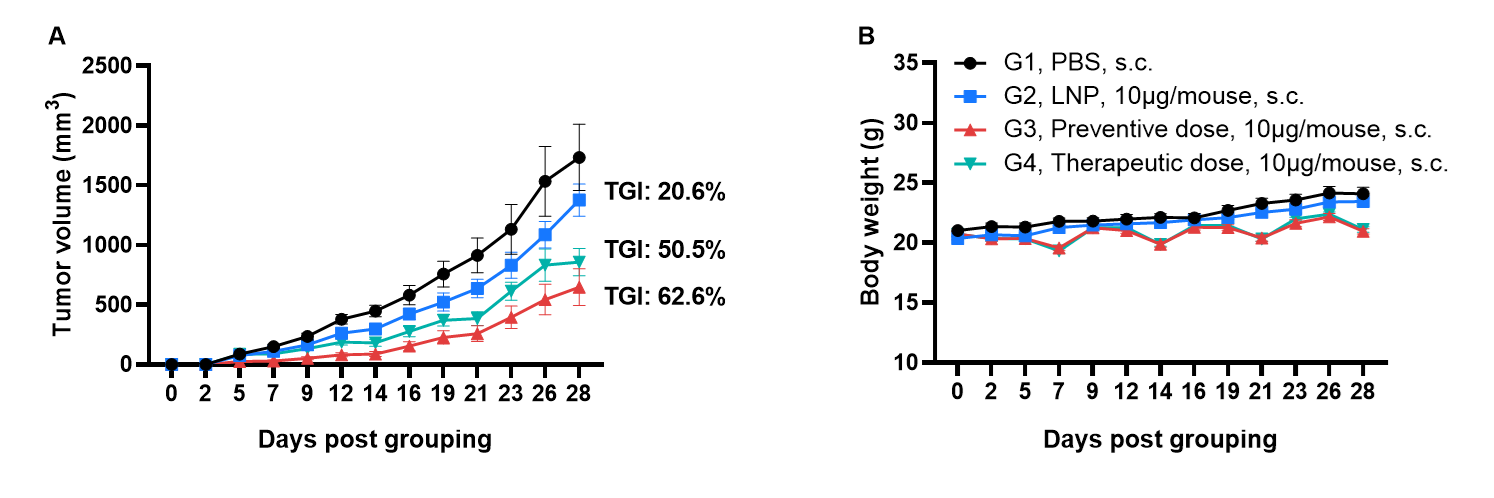
Antitumor activity of LMP2 mRNA vaccine against syngeneic tumors. (A) Antitumor activity of therapeutic treatment with mRNA vaccine. B-HLA-A2.1 mice (n = 8/group) were vaccinated with PBS, LNP or LNP-mRNA (provided by the client). B-HLA-A2.1 mice were implanted subcutaneously with 1×106 B-HLA-A2.1/hLMP2 MC38 tumor cells in the right flank and immunized at with PBS, LNP or mRNA vaccines via subcutaneous (s.c.) injection. As shown in panel A, the mRNA vaccines demonstrated robust inhibition of tumor growth and significantly prolonged overall survival compared to the control group. B. Body weight changes during tumor growth observation. These results demonstrate that B-HLA-2.1 mice provide a powerful preclinical model for in vivo evaluation of mRNA vaccines. Values are expressed as mean ± SEM.
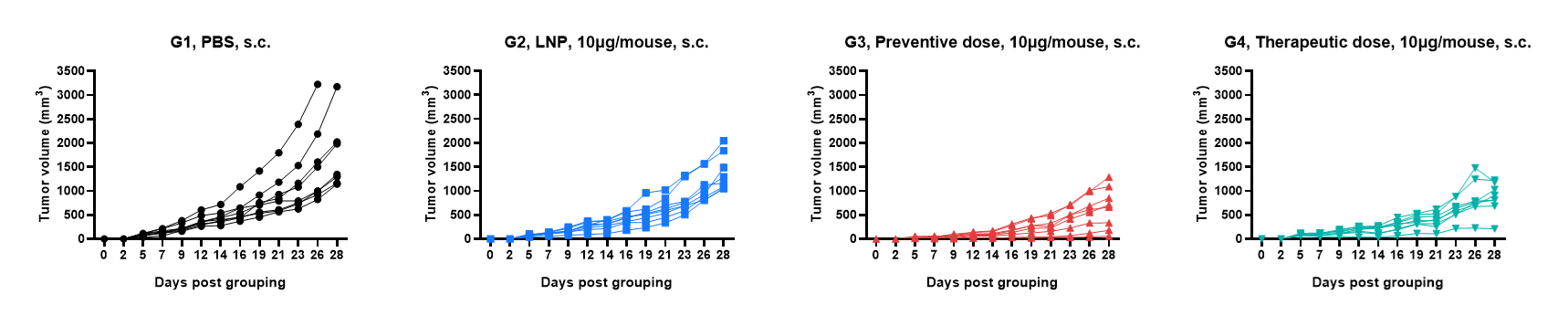
Antitumor activity of mRNA against syngeneic tumors. B-HLA-A2.1/hLMP2 MC38 tumor cells growth of individual mice.
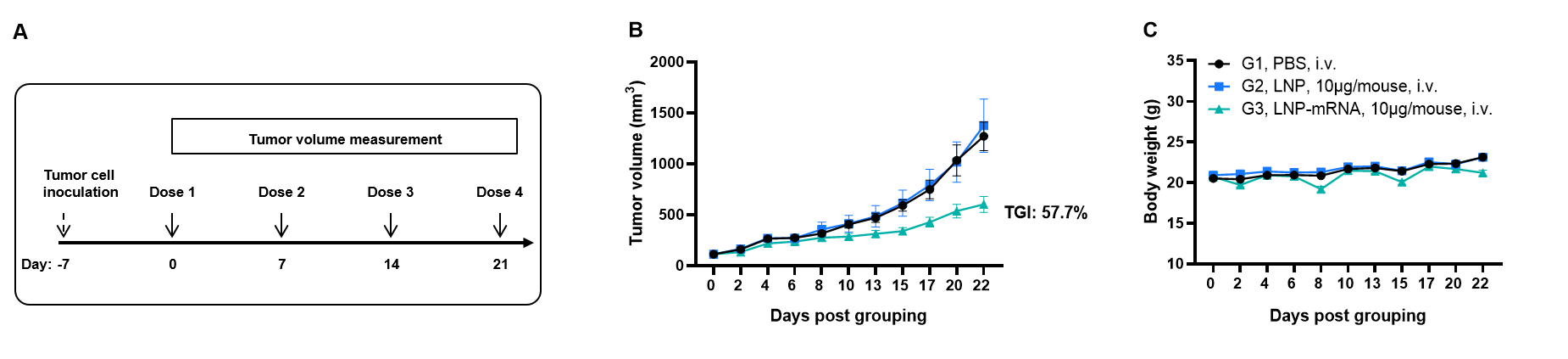
Antitumor activity of AFP mRNA vaccine against syngeneic tumors. (A) Experimental scheme. (B) Antitumor activity of therapeutic treatment with mRNA vaccine. B-HLA-A2.1 mice (n = 8/group) were vaccinated with PBS, LNP or LNP-mRNA. B-HLA-A2.1 mice were implanted subcutaneously with 1×106 B-HLA-A2.1/HPV16 E6/E7 MC38 tumor cells in the right flank and immunized four times at 1-week intervals with PBS, LNP or mRNA vaccines via intravenous (i.v.) injection on days 0, 7, 14 and 21 after tumor volume reached approximately 100 mm3. As shown in panel B, the mRNA vaccines demonstrated robust inhibition of tumor growth and significantly prolonged overall survival compared to the control group. C. Body weight changes during tumor growth observation. These results demonstrate that B-HLA-2.1 mice provide a powerful preclinical model for in vivo evaluation of mRNA vaccines. Values are expressed as mean ± SEM.
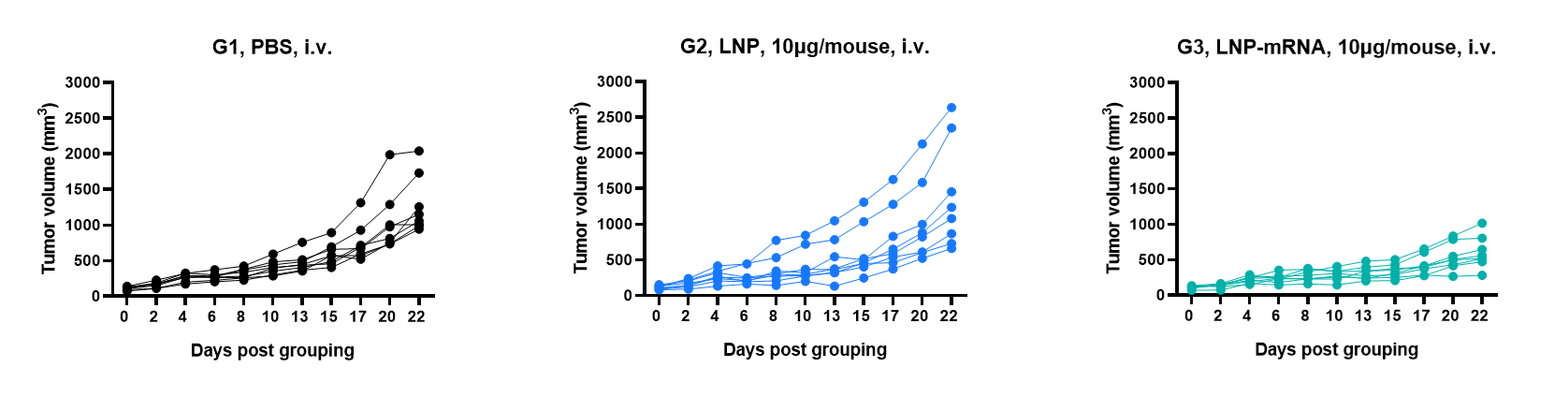
Antitumor activity of mRNA against syngeneic tumors. B-HLA-A2.1/HPV16 E6/E7 MC38 tumor cells growth of individual mice.