B-hDCIR mice
| Strain Name |
C57BL/6N-Dcirtm2(DCIR)Bcgen/Bcgen
|
Common Name | B-hDCIR mice |
| Background | C57BL/6N | Catalog number |
112352 |
|
Related Genes |
CD367, CLECSF6, DCIR, DDB27, HDCGC13P, LLIR, hDCIR |
||
|
NCBI Gene ID |
50856 | ||
- DCIR was first discovered in 1999, a novel member of the calcium-dependent (C-type) lectin family. DCIR is unique among known C-type lectin receptors on DC or neutrophils because it contains ITIM, a canonical immunoreceptor tyrosine-based inhibitory motif (ITIM) in the cytoplasmic region that transduces inhibitory signals by activating phosphatases SHP-1 and SHP-2 following activation. DCIR was expressed on DC, monocytes, macrophages, B lymphocytes, and granulocytes but not on NK or T cells.
- The exons 1-6 of mouse Dcir gene that encode extracellular domain, transmembrane domain, cytoplasmic region, and 3’UTR are replaced by human counterparts in B-hDCIR mice. The promoter and 5’UTR region of the mouse gene are retained. The human DCIR expression is driven by endogenous mouse Dcir promoter, while mouse Dcir gene transcription and translation will be disrupted.
- Human DCIR was detectable in DCs/macrophages/neutrophils of spleen, macrophages in blood, and DCs/monocytes/macrophages/neutrophils in bone marrow of homozygous B-hDCIR mice, but not in wild-type C57BL/6N mice.
- DCIR acts as an HIV-1 ligand and is engaged in the mechanisms that lead to productive viral infection. The dendritic cell immunoreceptor (DCIR), a C-type lectin receptor found on dendritic cells (DCs), serves as an attachment factor for HIV-1 and aids in HIV-1 transmission to CD4+ T lymphocytes (CD4TL).
- Blocking DCIR signaling ameliorates colitis and suppresses colonic tumors, suggesting DCIR as a possible target for the treatment of these diseases.
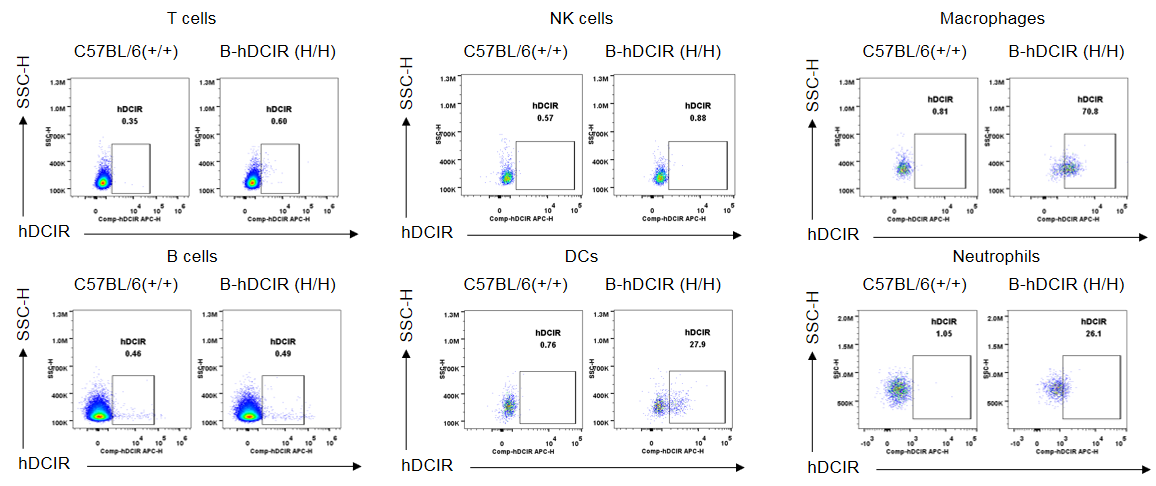
Strain specific DCIR expression analysis in wild-type C57BL/6N and homozygous B-hDCIR mice by flow cytometry. Splenocytes were collected from wild-type C57BL/6N mice (+/+) and homozygous B-hDCIR mice (H/H) and analyzed by flow cytometry with species-specific anti-DCIR antibody (R&D, FAB1748A). Human DCIR was detectable in spleen DCs, macrophages, and neutrophils of homozygous B-hDCIR mice, but not in wild-type C57BL/6N mice.
Protein expression analysis in Bone marrow
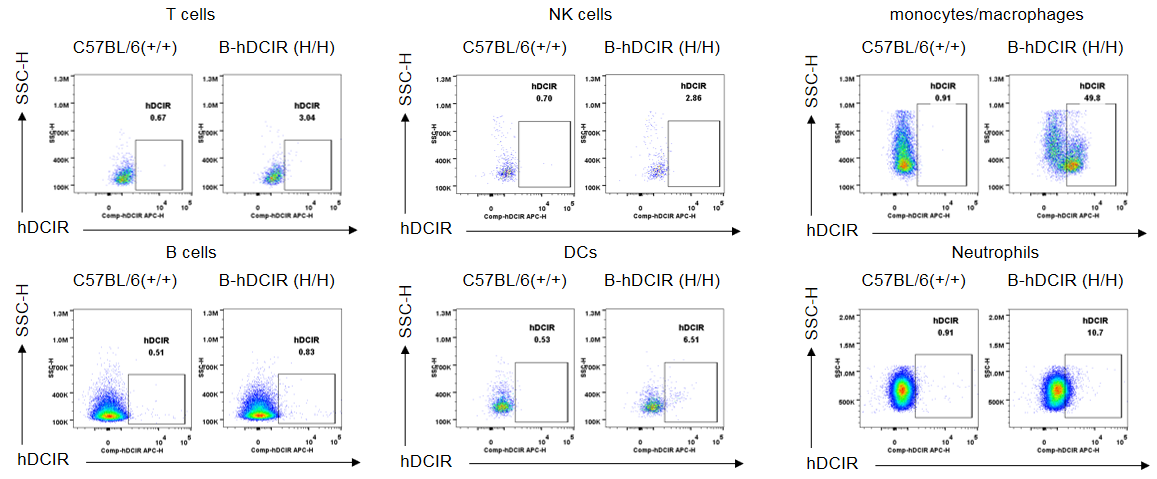
Strain specific DCIR expression analysis in wild-type C57BL/6N and homozygous B-hDCIR mice by flow cytometry. Bone marrow were collected from wild-type C57BL/6N mice (+/+) and homozygous B-hDCIR mice (H/H) and analyzed by flow cytometry with species-specific anti-DCIR antibody (R&D, FAB1748A). Human DCIR was detectable in Bone marrow DCs, monocytes/macrophages, and neutrophils of homozygous B-hDCIR mice, but not in wild-type C57BL/6N mice.
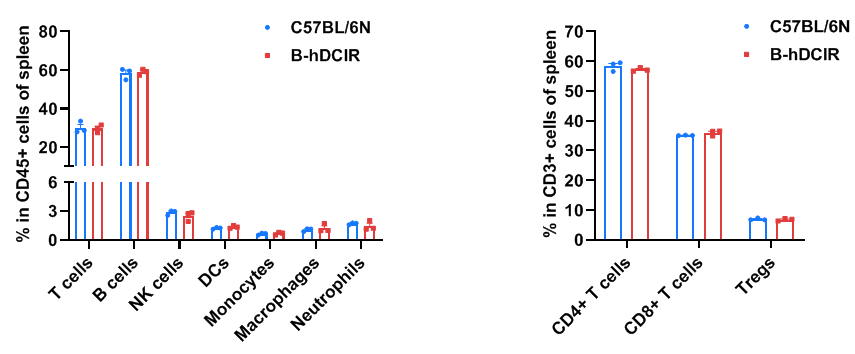
Frequency of leukocyte subpopulations in spleen by flow cytometry. Splenocytes were isolated from wild-type C57BL/6N mice and homozygous B-hDCIR mice (female, 6-week-old, n=3). A. Flow cytometry analysis of the splenocytes was performed to assess the frequency of leukocyte subpopulations. B. Frequency of T cell subpopulations. Frequencies of T cells, B cells, NK cells, DCs, monocytes, macrophages, neutrophils, CD4+ T cells, CD8+ T cells and Tregs in B-hDCIR mice were similar to those in C57BL/6N mice, demonstrating that humanization of DCIR does not change the frequency or distribution of these cell types in spleen. The frequency of leukocyte subpopulations in lymph node and blood of B-hDCIR mice were also comparable to wild-type C57BL/6N mice (Data not shown). Values are expressed as mean ± SEM. Significance was determined by two-way ANOVA test. *P < 0.05, **P < 0.01, ***P < 0.001.









