|
Strain Name
|
C57BL/6-Egfrtm2(EGFR)Bcgen/Bcgen
|
Common Name
|
B-hEGFR mice
|
|
Background
|
C57BL/6
|
Catalog number
|
120771
|
|
Aliases
|
EGFR, ERBB, ERBB1, ERRP, HER1, NISBD2, PIG61,
mENA, epidermal growth factor receptor
|
mRNA expression analysis
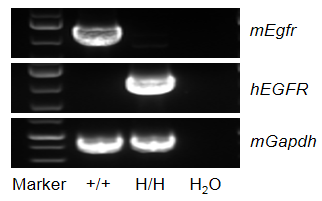
Strain specific analysis of EGFR gene expression in wild type (WT) mice and B-hEGFR mice by RT-PCR. Mouse Egfr mRNA was detectable only in liver of WT mice (+/+). Human EGFR mRNA was detectable only in homozygous B-hEGFR mice (H/H) but not in WT mice (+/+).
IHC analysis of EGFR expression
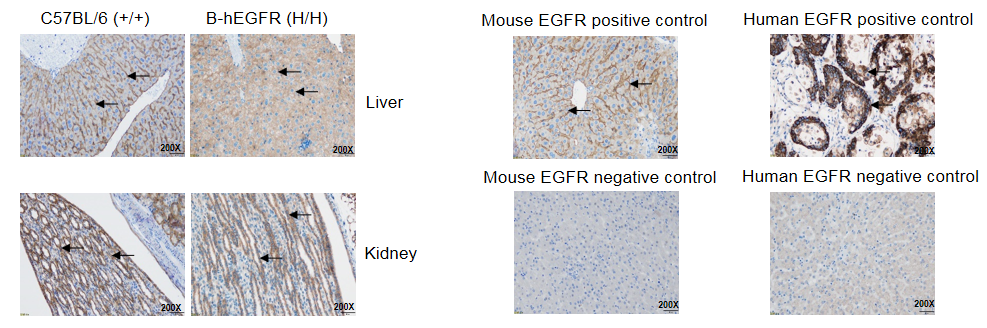
Immunohistochemical (IHC) analysis of EGFR expression in homozygous B-hEGFR mice. The liver and kidney were collected from WT and homozygous B-hEGFR mice (H/H) and analyzed by IHC with anti-EGFR antibody. Mouse EGFR was detectable in WT mice, and human EGFR was detectable in homozygous B-hEGFR mice. The arrow indicates tissue cells with positive EGFR staining (brown).
Protein expression profile of EGFR
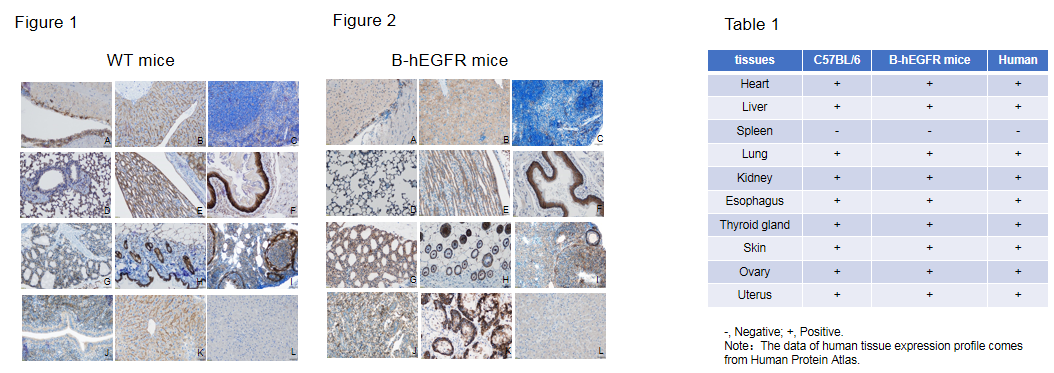
Immunohistochemical (IHC) analysis of EGFR protein expression in wild-type (WT) mice and B-hEGFR mice. Ten major tissues were collected from WT mice and homozygous B-hEGFR mice (2 females, 8 weeks-old), and analyzed by IHC with anti-EGFR antibodies. Humanized EGFR was detected in the heart (A), liver (B), lung (D), kidney (E), esophagus (F), thyroid (G), skin (H), ovary (I) and uterus (J) of homozygous B-hEGFR mice (Figure 2) and human EGFR positive control (K), but not in spleen (C) and human EGFR negative control (L). This is similar to the mouse EGFR expression profile of WT mice (Figure 1, Table 1). The results showed that humanized EGFR did not change the expression site of EGFR protein in B-hEGFR mice, and the expression profile of EGFR in B-hEGFR mice was similar to that in normal human tissues (Table 1).
Hematology analysis
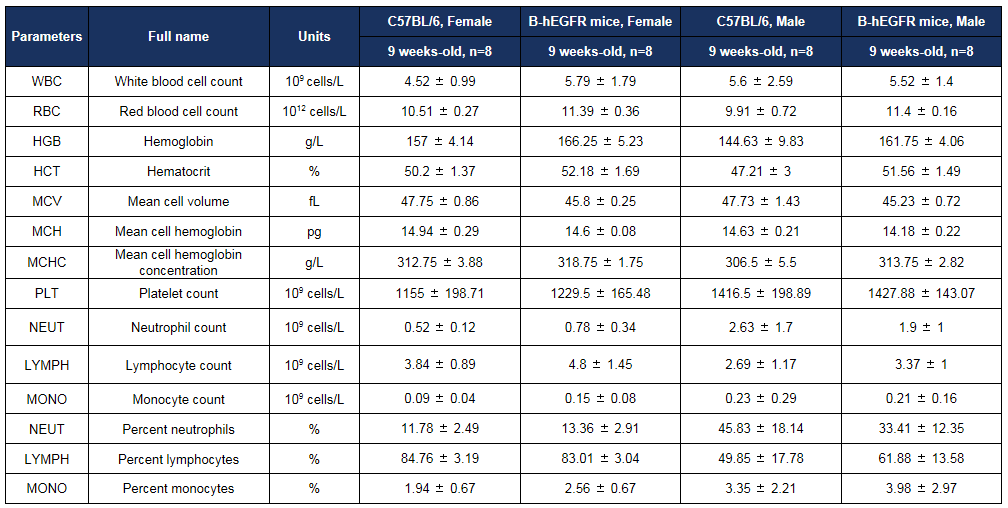
Complete blood count (CBC) of B-hEGFR mice. Values are expressed as mean ± SD.
Biochemistry analysis
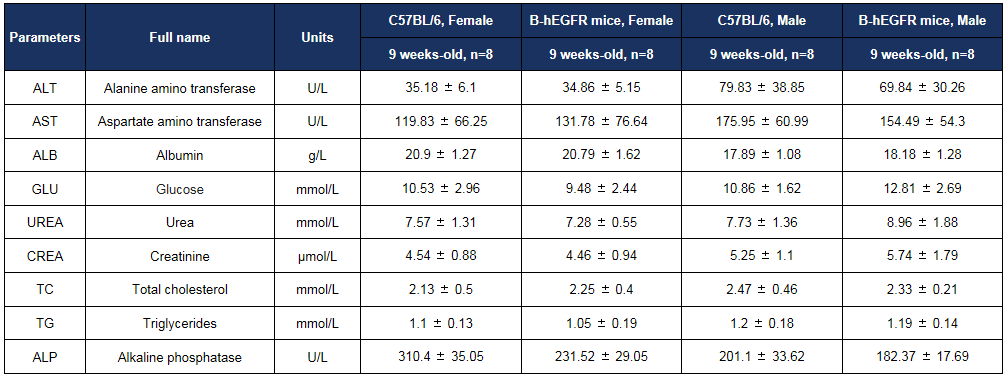
Biochemical test of B-hEGFR mice. Values are expressed as mean ± SD.
In vivo efficacy of anti-human EGFR antibodies
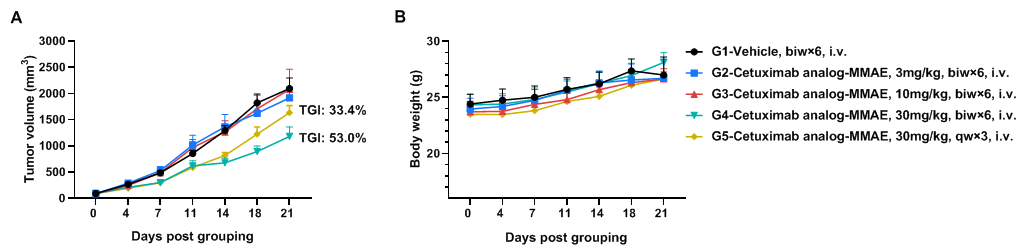
Antitumor activity of anti-human EGFR antibody in B-hEGFR mice. (A) Anti-human EGFR antibody inhibited B-Tg(hEGFR) MC38 tumor growth in homozygous B-hEGFR mice. Murine colon cancer B-Tg(hEGFR) MC38 cells were subcutaneously implanted into homozygous B-hEGFR mice (female, 6-8 weeks-old, n=6). Mice were grouped when tumor volume reached approximately 50-150 mm3, at which time they were intravenous injected with anti-human EGFR ADC cetuximab analog-MMAE (in house) indicated in panel. (B) Body weight changes during treatment. As shown in panel A, 30mg/kg anti-human EGFR ADC cetuximab analog-MMAE (in house) treatment group was efficacious in controlling tumor growth in B-hEGFR mice, demonstrating that the B-Tg(hEGFR) MC38 provide a powerful preclinical model for in vivo evaluation of anti-human EGFR antibodies. Values are expressed as mean ± SEM.
In vivo efficacy of anti-human EGFR antibodies-individual tumor growth curves
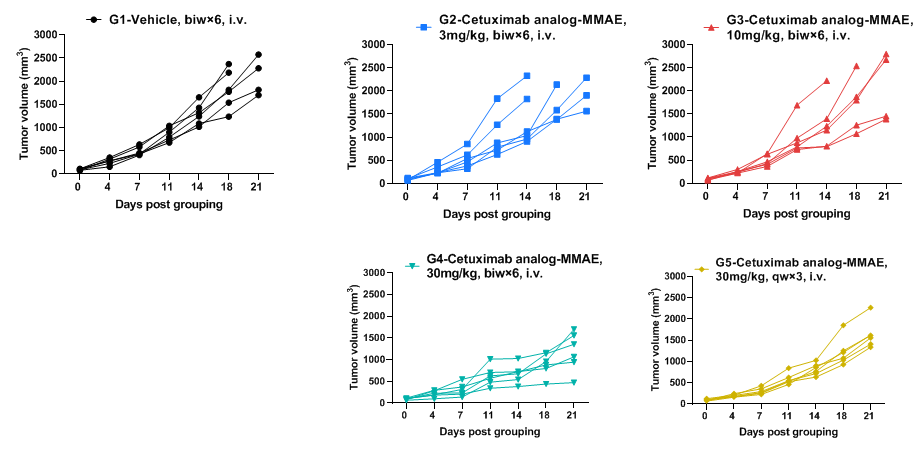
Antitumor activity of anti-human EGFR antibody in B-hEGFR mice. Anti-human EGFR antibody inhibited B-Tg(hEGFR) MC38 tumor growth in homozygous B-hEGFR mice. Murine colon cancer B-Tg(hEGFR) MC38 cells were subcutaneously implanted into homozygous B-hEGFR mice (female, 6-8 weeks-old, n=6). Mice were grouped when tumor volume reached approximately 50-150 mm3, at which time they were intravenous injected with anti-human EGFR ADC cetuximab analog-MMAE (in house) indicated in panel.
Toxicity analysis of anti-human EGFR antibody in B-hEGFR mice
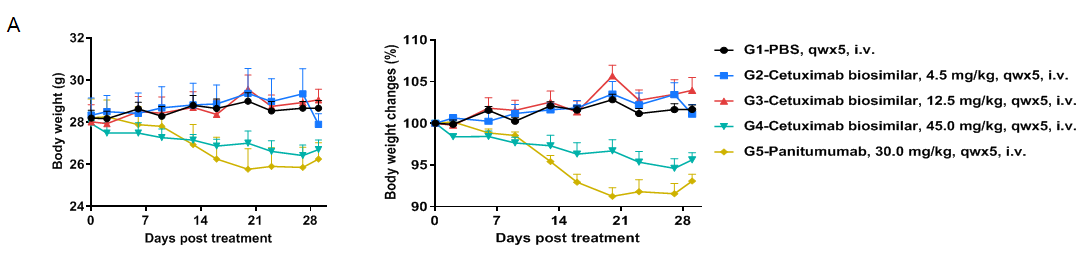
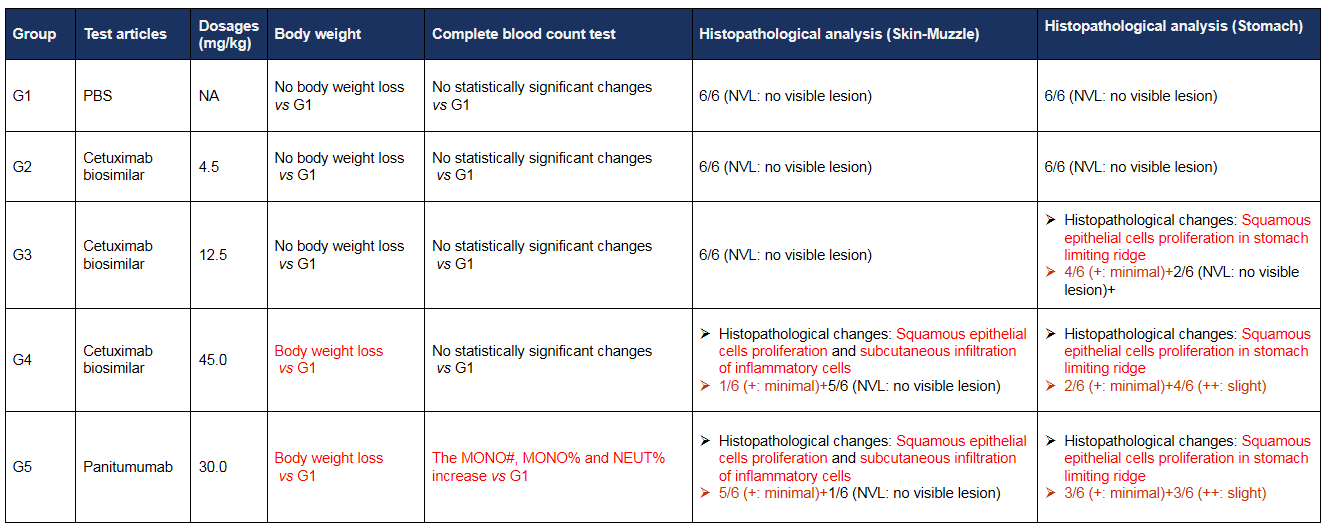
Anti-human EGFR antibody cetuximab biosimilar (Bio X Cell, 907623J2) or panitumumab (Takeda, 549661) were intravenously injected into B-hEGFR mice (male, 14-15 weeks-old, n=6). Mice were weighed twice a week, and their condition was observed daily. At the end of the experiment, blood samples were collected for complete blood count test. Additionally, tissue samples were collected from the nasal and oral skin, abdominal skin, stomach, duodenum, jejunum, ileum, cecum, colon, and rectum, and then subjected to pathological analysis. (A) Body weight and body weight changes during treatment. The results showed that 45.0 mg/kg cetuximab biosimilar and 30.0 mg/kg panitumumab resulted in significant weight loss in B-hEGFR mice. Values are expressed as mean ± SEM.
Note: This experiment is a collaboration with the client.
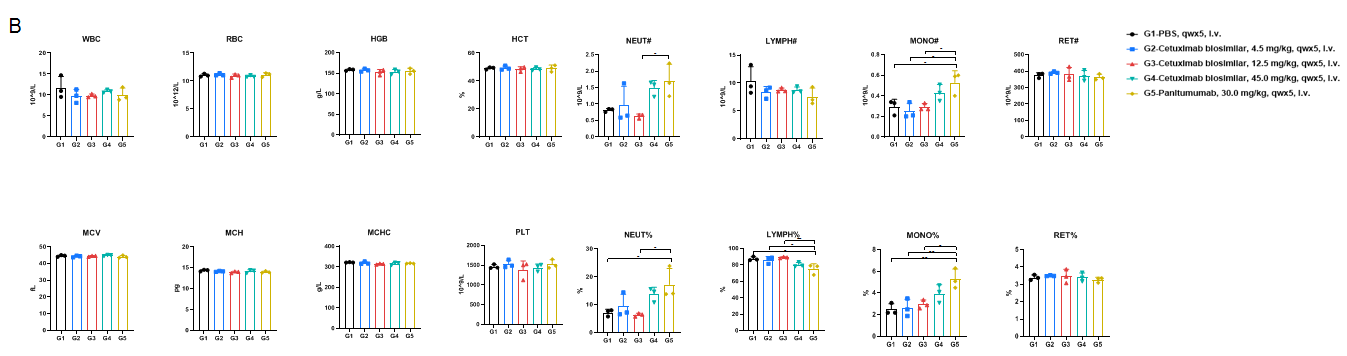
Anti-human EGFR antibody cetuximab biosimilar (Bio X Cell, 907623J2) or panitumumab (Takeda, 549661) were intravenously injected into B-hEGFR mice (male, 14-15 weeks-old, n=6). Mice were weighed twice a week, and their condition was observed daily. At the end of the experiment, blood samples were collected for complete blood count test. Additionally, tissue samples were collected from the nasal and oral skin, abdominal skin, stomach, duodenum, jejunum, ileum, cecum, colon, and rectum, and then subjected to pathological analysis. (B) Complete blood cell count detection at the endpoint of the experiment. 45.0 mg/kg cetuximab biosimilar and 30.0 mg/kg panitumumab resulted in an increase in the number of neutrophils and monocytes, while there were no significant changes in blood parameters in the other treatment groups. Values are expressed as mean ± SD. Significance was determined by one-way ANOVA test. *p<0.05, **p<0.01.
Note: This experiment is a collaboration with the client.
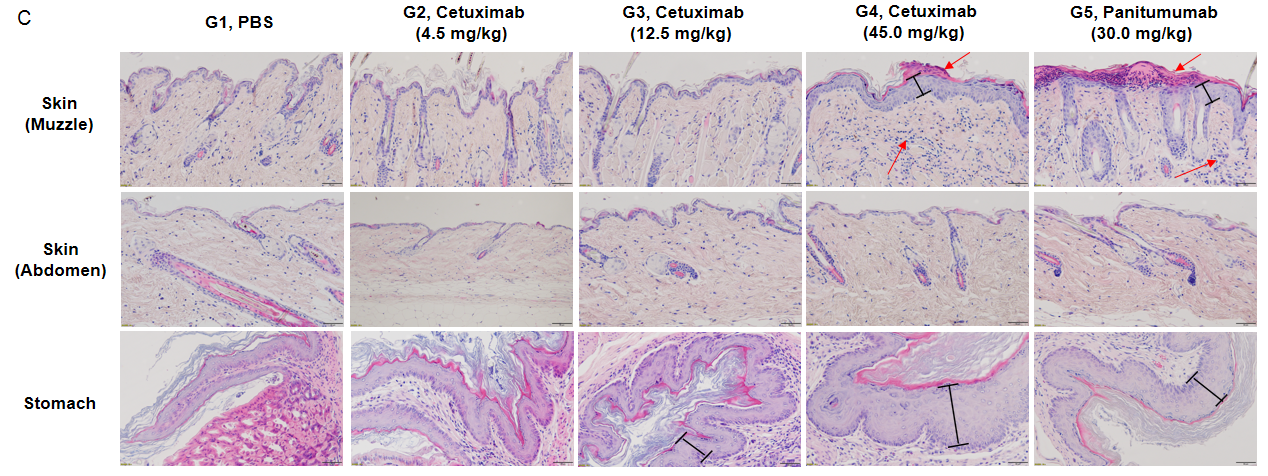
(C) Pathological diagrams of various tissues. The histopathological examination in muzzle skin revealed article-related alteration in cetuximab biosimilar 45 mg/kg dose group (1/6, minimal), which showed squamous epithelial cells proliferation and subcutaneous infiltration of inflammatory cells. The similar article-related changes were also found in panitumumab group (5/6, minimal). Squamous epithelial cells proliferation in stomach limiting ridge were observed in mice administrated cetuximab biosimilar at the dose of 12.5 mg/kg (4/6, minimal) and 45 mg/kg groups (2/6, minimal; 4/6, slight), which were considered test-article and dose related. The similar article-related changes were also found in panitumumab group (3/6, minimal; 3/6, slight). Neither cetuximab biosimilar nor panitumumab showed any toxicity in the abdominal skin, duodenum, jejunum, ileum, cecum, colon, and rectum of mice (The data is not shown).
Note: This experiment is a collaboration with the client.
Toxicokinetic analysis of anti-human EGFR antibody in B-hEGFR mice
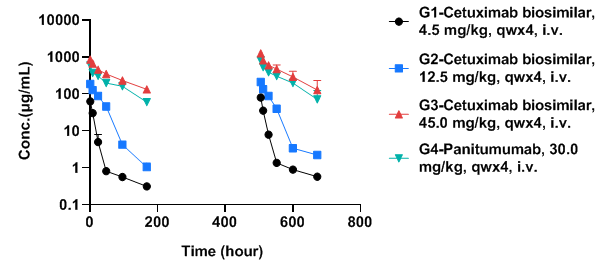
Anti-human EGFR antibody cetuximab biosimilar (Bio X Cell, 907623J2) or panitumumab (Takeda, 549661) were intravenously injected into B-hEGFR mice (male, 14-15 weeks-old, n=2). Blood samples were collected at 1h, 8h, 24h, 48h, 96h, and 168h after the first and fourth doses, and then the drug concentrations in the blood were measured. As shown in the figure, with the increase of dosage, the blood concentration of cetuximab biosimilar also increases. This indicates that the relationship between the blood concentration of cetuximab biosimilar and dosage is linear within the experimental dosage range. The existence of this linear relationship may be due to the absence of saturation during the absorption, distribution, metabolism, and excretion of drugs in mice.
Note: This experiment is a collaboration with the client.





















