Biocytogen’s antibody BD and licensing team is pleased to attend and host 1-on-1 meetings at the 17th annual BIO-Europe Spring, taking place March 20–22, 2023 in Basel, Switzerland.
With the successful launch of Biocytogen’s proprietary fully human antibody mice (RenMabTM, RenLite® and RenNanoTM), Biocytogen has developed 6 fully human antibody discovery platforms for development of monoclonal antibodies, bispecific and multi-specific antibodies, nanobodies, bispecific ADCs, TCR-mimic antibodies against intracellular TAAs, and antibodies against GPCRs and other challenging targets.
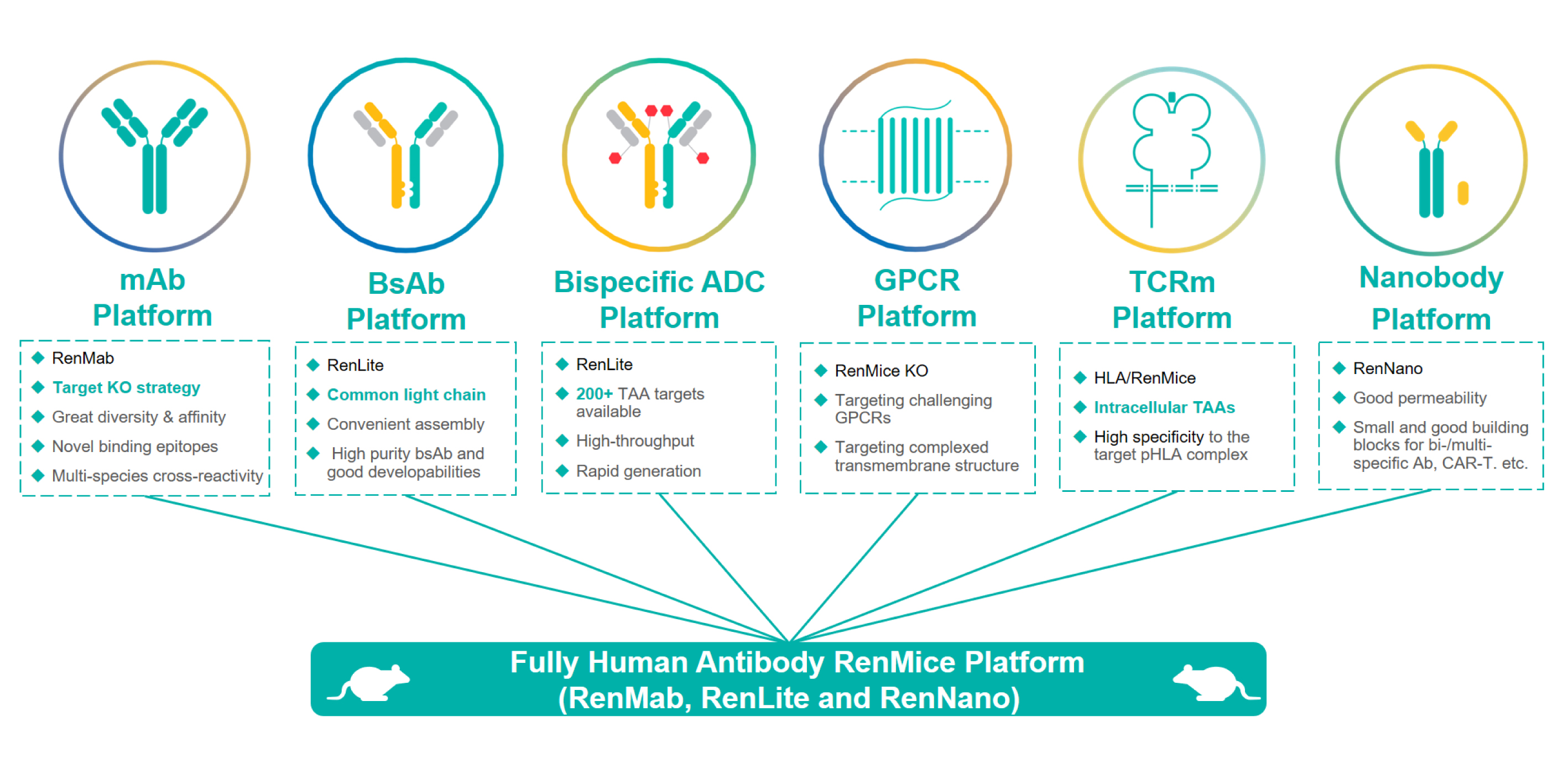
Since the launch of Project Integrum, an initiative to develop therapeutic antibodies for more than 1000+ targets, the company has generated:
5 clinical stage assets:
- YH003: CD40 mAb in phase II MRCTs;
- YH001: CTLA-4 mAb in an ongoing phase I clinical trial for the treatment of sarcoma in partnership with TRACON;
- YH008: PD-1×CD40 bsAb, which received both FDA and NMPA IND clearance;
- YH002: OX40 mAb in phase I clinical trials in Australia and China;
- YH004: 4-1BB mAb in phase I clinical trials in Australia and China.
Numerous preclinical assets, including:
- 10 fully human bispecific antibodies (BsAbs): YH006, a fully human CTLA-4×OX40 BsAb generated by the RenLite platform;
- 20+ bispecific antibody-drug conjugates (BsADCs): YH012, a HER2 x TROP2 BsADC and YH013, an EGFR x cMET BsADC; 40+ TAA-targeting backbones for plug and play.
- 10+ TCR-mimic (TCRm) antibodies against intracellular tumor-associated antigens (TAAs), including WT-1, KRAS, P53, AFP, GP100、NY-ESO-1, etc;
- 30+ monoclonal antibodies for novel targets: B7H3, TNFR2, CCR8, Siglec-15, AMHR2 and other novel TAAs, co-stimulatory/co-inhibitory molecules, tumor microenvironment molecules, and GPCRs, among others;
- 600+ antibody hits: targeting B7H4, DLL3, MUC1, GPRC5D, PSMA, LRRC15 and other therapeutic targets for oncology, inflammatory and autoimmune diseases, infectious diseases, and neurodegenerative disease applications.
Biocytogen has reached drug co-development agreements and RenMabTM/RenLite® licensing agreements with companies around the world, including Janssen, Merck KGaA, ADC Therapeutics, Xencor, TRACON, BeiGene, Remegen, and Chipscreen. The company will continue to actively seek partnerships to accelerate the development of novel therapies. To schedule a 1-on-1 meeting with Biocytogen, please contact us at BD-Licensing@biocytogen.com.
About BIO-EUROPE SPRING
Over the years, BIO-Europe Spring has become Europe’s largest springtime partnering event. Its international reach makes it a one of a kind offering and gateway to the global life science community. The event will cater to the needs of the entire value chain, start-up and innovator educational programmes, industry trends and outlooks from KOLs, company pitches, professional partnering meetings as well as various networking opportunities are part of the ROI proven feature set. BIO-Europe Spring is produced by EBD Group, the leading partnering firm for the global biotechnology industry, with the support of the Biotechnology Innovation Organization (BIO).
About Biocytogen
Biocytogen (HKEX: 02315) is a global biotechnology company that drives the research and development of novel antibody-based drugs with innovative technologies. Using its proprietary RenMabTM/RenLite®/RenNanoTM mice platforms for fully human monoclonal, bispecific/multispecific antibody and nanobody development, Biocytogen has integrated its in vivo drug efficacy screening platforms and strong clinical development expertise to streamline the entire drug development process. Biocytogen is undertaking a large-scale project to develop first-in-class and/or best-in-class antibody drugs for more than 1000 targets, known as Project Integrum (RenMiceTM HiTS Platform). As of June 30, 2022, this project has resulted in 28 drug co-development agreements and 16 RenMiceTMlicensing agreements with companies around the world, including several partnerships with multinational pharmaceutical companies (MNCs). Biocytogen's pipeline includes 12 core products, among which two products are in phase II multi-regional clinical trials and two products are in phase I. Headquartered in Beijing, Biocytogen has branches in Haimen Jiangsu, Shanghai, Boston, USA and Heidelberg, Germany. For more information, please visit http://en.biocytogen.com.cn. Follow Biocytogen on LinkedIn, and on Twitter @Biocytogen_Ab.
Biocytogen’s Contacts
Antibody assets and platforms: BD-Licensing@biocytogen.com
Media: pr@bbctg.com.cn









