Biocytogen (HKEX: 02315) will attend the upcoming 41st J.P. Morgan (JPM) Healthcare Conference week, and participate in several satellite conferences, including the Biotech Showcase and BIO Partnering at JPM, which are all to be held in San Francisco from January 9-12, 2023. The team will also present in the BFC conference and ACCESS CHINA before and after JPM week. Dr. Chaoshe Guo, VP of Biocytogen, and the antibody drug Business Development & Licensing (BDL) team will be in attendance to discuss Biocytogen’s innovative antibody technology platforms and antibody drug assets to explore partnerships with other pharmaceutical and biotech companies in attendance. The team’s full itinerary during JPM week is as follows:
- Jan. 8, The 6th BFC Annual Global Healthcare Business Development and Investment Conference (Presentation)
- Jan. 9-12, 41st Annual J.P. Morgan Healthcare Conference
- Jan. 9-11, Biotech Showcase (Presentation)
- Jan. 9-12, BIO Partnering at JPM
- Jan. 16-20, ACCESS CHINA Biotech Forum @ JPM 2023 (Presentation)


Dr. Vivian Tian, Senior Director of the BDL team, will give a presentation at the Biotech Showcase, where she will introduce Biocytogen’s 6 therapeutic antibody discovery platforms for various targets and modalities, the company’s large-scale in vivo evidence-based drug efficacy screening platform, and the antibody-based drug assets generated by these platforms, including preclinical mAb/BsAb/BsADC assets and clinical assets. Details are summarized as follows:
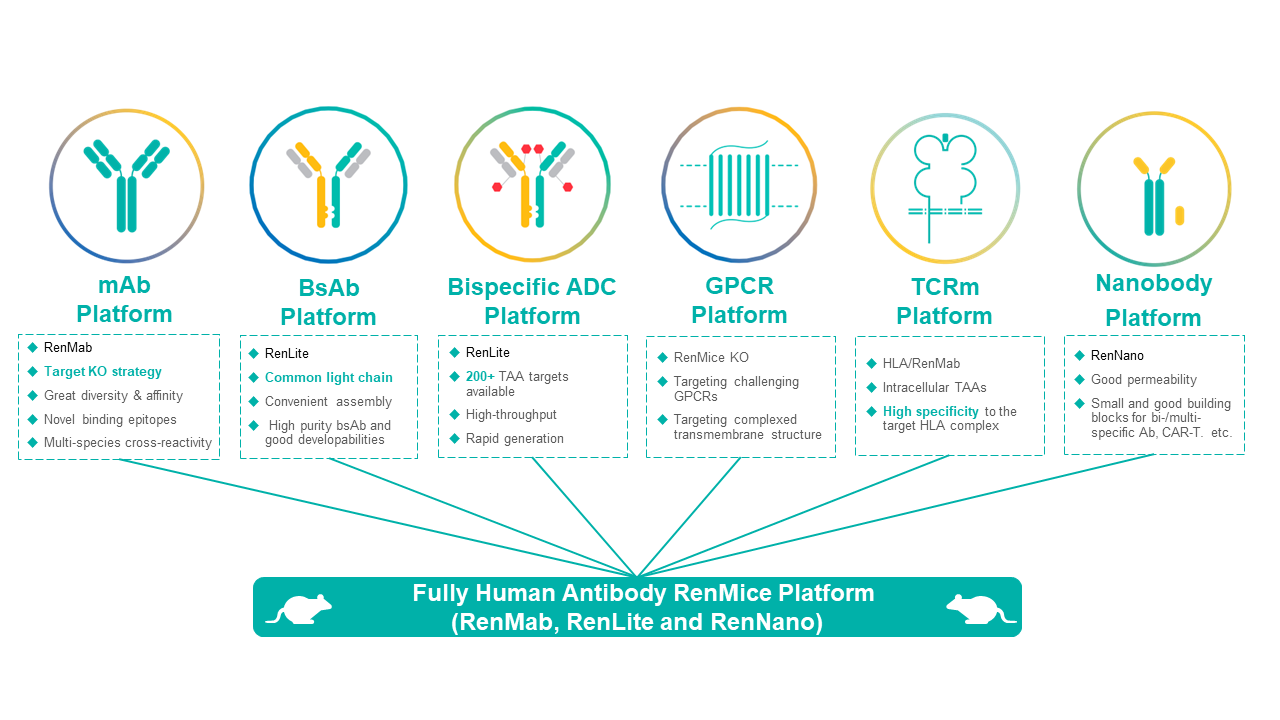
1) 6 innovative fully human antibody discovery platforms for various targets and modalities:
- mAb platform: RenMab-based monoclonal antibody discovery with high affinity and great diversity;
- BsAb platform: RenLite-based common light chain bispecific antibody discovery with high pairing success rate and good druggability;
- Bispecific ADC platform: High-throughput RenLite-based bispecific ADC discovery for 200+ TAA targets with good tumor specificity, anti-tumor activity, and CMC efficiency;
- GPCR platform: Target knockout RenMice-based antibody discovery for multi-species cross-regonition of GPCR and other challenging targets;
- TCRm antibody platform: HLA-transgenic RenMice-based TCRmimic (TCRm) antibody divscovery for intracellular targets with superior affinity and specificity;
- Nanobody platform: RenNano-based single-domain antibody (nanobody) discovery with great blood-brain-barrier permeability and tumor infiltrating ability, suitable for the assembly of bispecific, multi-specific antibodies, CAR-T therapies, etc.
2) Preclinical mAb/BsAb/BsADC assets:
In the past two years, Biocytogen has been continuously generating competitive antibody candidates or hits through Project Integrum, which can be further developed into various drug modalities, including mAb, BsAb, BsADC, TCR-mimic antibody and nanobody drugs. Our leading candidates to be discussed are as follows:
- 10 fully human BsAbs: YH006, a fully human CTLA-4×OX40 BsAb generated by the RenLite platform;
- 20+ bispecific ADCs: YH012, a HER2 x TROP2 BsADC and YH013, an EGFR x cMET BsADC, both with expected IND submissions in 2023;
- 10+ TCRm antibodies: Targeting intracellular TAAs including WT-1, KRAS, P53, AFP, GP100、NY-ESO-1, etc;
- 30+ mAbs for novel targets: targeting B7H3, TNFR2, CCR8, AMHR2 and other novel TAAs, co-stimulatory/co-inhibitory molecules, tumor microenvironment molecules, GPCRs, etc;
- 600+ antibody hits: targeting B7H4, DLL3, MUC1, GPRC5D, PSMA, LRRC15 and other therapeutic targets for oncology, inflammatory and autoimmune diseases, infectious diseases, and neurodegenerative disease applications.
3) Clinical-stage assets
Five assets from Biocytogen’s pipeline have entered clinical trials. All of them were obtained from our high-throughput in vivo efficacy-based screening platform and demonstrated good efficacy and safety.
- YH003: CD40 mAb in phase II MCRTs;
- YH001: CTLA-4 mAb in an ongoing phase I clinical trial for the treatment of sarcoma in partnership with TRACON;
- YH008: PD-1×CD40 bsAb, which received FDA IND clearance in December 2022;
- YH002: OX40 mAb in phase I clinical trials in Australia and China;
- YH004: 4-1BB mAb in phase I clinical trials in Australia and China.
With our innovative antibody technology platforms and drug assets developed by these unique platforms, Biocytogen has established collaborations with many biopharmaceutical and biotech companies, including Merck KGaA, ADC therapeutics, Xencor, Tracon, BeiGene, Remegen, etc. We are excited to continue expanding our global partnerships to accelerate new drug development.
Contact us at BD-Licensing@biocytogen.com to set up a meeting. We look forward to discussing licensing and co-development opportunities with you!









