AD Model Introduction
Atopic dermatitis (AD) is a chronic skin disease with the main symptoms of itching, erythema and squamous lesions, cardiovascular, mental disorders (depression, anxiety, sleep disorders, etc.) and other diseases. In order to better study atopic dermatitis, researchers have developed a variety of animal models of atopic dermatitis on mice, including (1) AD models caused by enhanced skin sensitization using epithelial sensitive antigens, haptens, etc.; (2) genetically engineered mouse AD models; (3) spontaneous AD mouse models.
Biocytogen uses C57BL/6 wild mice and B-hIL4/hIL4R double gene humanized mice on C57BL/6 background to produce dermatitis lesions using oxazolone (OXA) sensitization and continuous challenge to establish AD disease models for efficacy evaluation of AD-related drugs1, 2.
Establishment of mouse model of AD
Experimental Animals:C57BL/6,7 weeks old, female
Modeling reagent:OXA
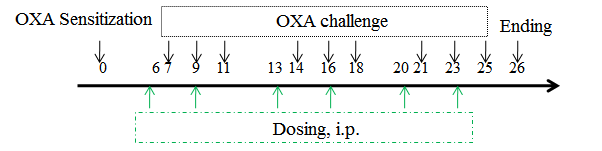
Modeling method:Sensitization—0 days, right ear application
Challenge—7-25 days, right ear application
Clinical assessment
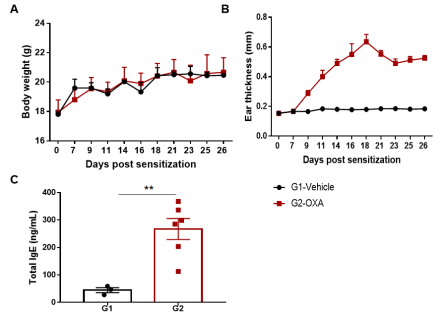
The AD model was induced by OXA in wild-type C57BL/6 mice. (A) Mouse body Weight. (B) Ear thickness. (C) Serum total IgE concentration. Ear thickness and the serum IgE were significantly increased in the OXA -treated group.
Histologic Analysis
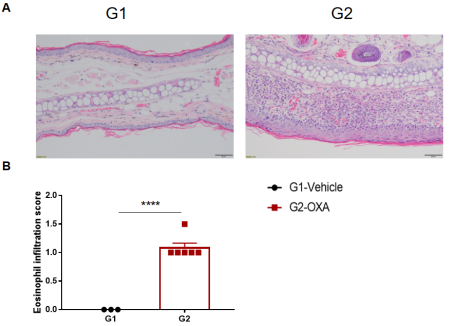
Analysis of ear skin pathology and lymphocyte infiltration in a wild-type C57BL/6 AD model. (A) Hematoxylin and eosin (H&E) staining of mouse ear tissue sections; (B) Eosinophil infiltration scores in the ear epidermis. The OXA-treated group showed different degrees of stromal cell proliferation, thickening, hyperkeratosis with hypokeratosis, crust, mixed inflammatory cell infiltration in dermis and subcutaneous tissue and other atopic dermatitis-related pathological changes in the epidermis. The infiltration score of eosinophils was significantly higher. The above results demonstrated that OXA could successfully induce AD-like pathology in wild-type C57BL/6 mice.
Efficacy Validation on AD(wild-type C57BL/6) Mouse Models for dexamethasone
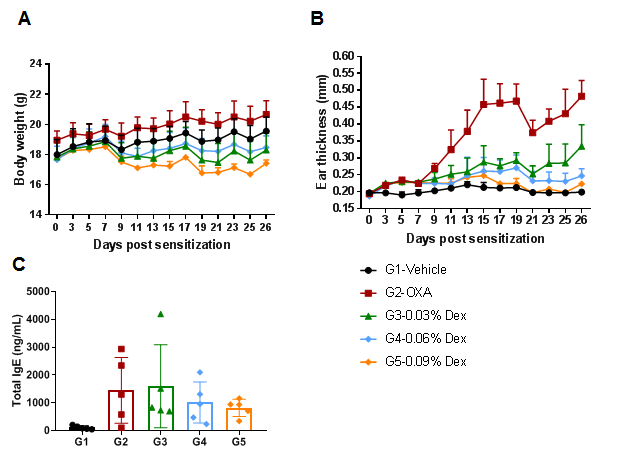
The efficacy of dexamethasone (Dex) was verified in a wild-type C57BL/6 AD model. (A) Mouse body weight. (B) Ear thickness. The results showed that different concentrations of dexamethasone could significantly reduce ear thickness compared with the OXA-only group. The high-dose group (0.09% Dex) completely relieved ear skin edema to the same level as controls. However, it was also associated with significant weight loss compared with the control group. (C) Serum total IgE concentration. The results showed that dexamethasone in G4 medium dose group (0.06% Dex) and G5 high dose group (0.09% Dex) could reduce the concentration of serum total IgE.
Histologic analysis of AD-like Disease
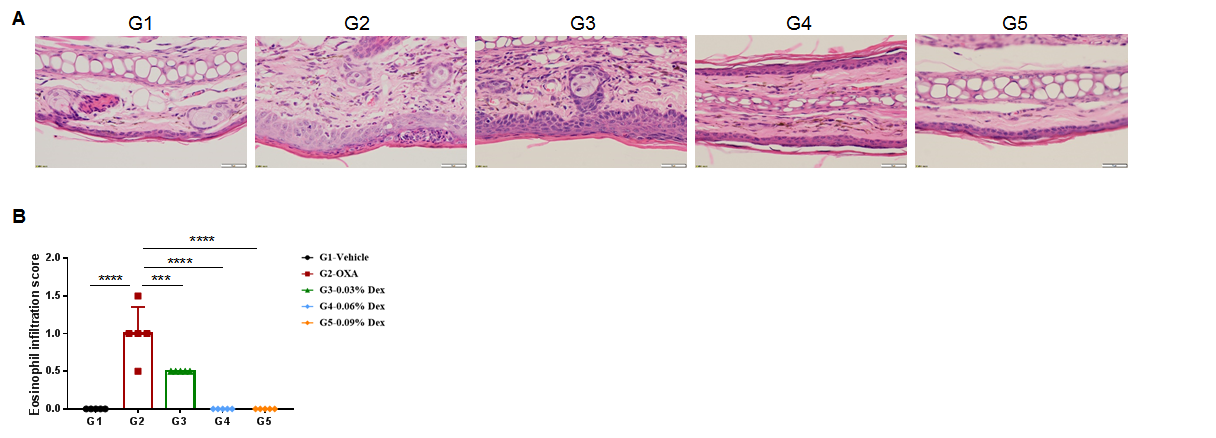
The efficacy of dexamethasone was verified using wild-type AD model. (A) Hematoxylin and eosin (H&E) staining of mouse ear tissue sections. (B) Eosinophil infiltration score of the ear epidermis. The results showed that pathological changes related to atopic dermatitis, such as stromal cell proliferation, thickening, and mixed inflammatory cell infiltration, were observed in the skin epidermis of the ears of animals in the OXA-treated group (G2), and these phenotypes were significantly alleviated in the Dex-treated groups (G3-G5) compared with the G2 modeling group, especially in the high dose group (0.09% Dex), which returned to baseline. The eosinophil infiltration score in the skin was significantly lower in the G3-G5 group than in the G2 group. Overall, dexamethasone improved atopic dermatitis-related symptoms, with Dex at concentrations of 0.06% and 0.09% having improved efficacy. The above results showed that the AD mouse model could be successfully induced for efficacy evaluation using OXA.
A mouse model of AD (B-hIL4/hIL4R mice) was used to evaluate the efficacy of the anti-human IL4RA antibody dupilumab
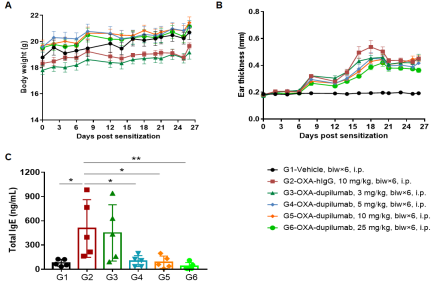
The efficacy of the anti-human IL4RA antibody dupilumab (in-house) was verified using an AD model generated in B-hIL4/hIL4RA mice. Mice were treated with different doses of dupilumab and changes in various parameters were detected. (A) Mouse Weight. (B) Ear epidermal thickness measurement. The results showed that different concentrations of dupilumab could relieve ear skin edema compared with the G2 modeling group. (C) Total IgE concentration in serum. Different concentrations of dupilumab significantly reduced serum total IgE concentrations. Ear thickness and serum total IgE concentrations were inversely correlated with antibody dose.
Histologic Assessment of AD in Humanized Mice
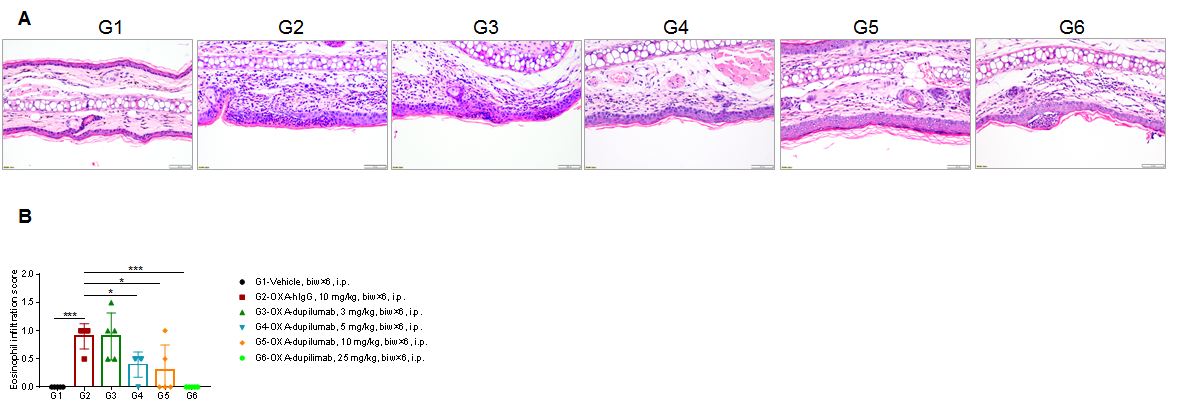
Efficacy of anti-human IL4RA antibody dupilumab (in-house) on skin and lymphocyte infiltration in the ear of B-hIL4/hIL4RA mice with experimental AD.(A)Hematoxylin and eosin (H&E) staining of mouse ear tissue sections. (B) Eosinophilic infiltration score of ear skin. The results showed that pathological changes related to atopic dermatitis, such as stromal cell proliferation, thickening, and mixed inflammatory cell infiltration, were observed in the ear skin epidermis of animals in the G2 (OXA + IgG) group, and the pathological changes of the skin diseases were significantly alleviated in the G3-G6 (dupilumab) groups compared with the G2 modeling group. The eosinophil infiltration score in the skin was significantly decreased in the G3-G5 group compared with the G2 group, in a dose-dependent manner. The above results indicate that B-hIL4/hIL4RA mice provide a powerful preclinical model for in vivo efficacy evaluation of anti-human IL4RA antibodies.
Product list
|
Product name |
Product number |
|
B-hIL4/hIL4RA mice |
120551 |
References
1. Vatrella, A., Fabozzi, I., Calabrese, C., Maselli, R. & Pelaia, G. Dupilumab: a novel treatment for asthma. J Asthma Allergy 7, 123-130 (2014).
2. Gandhi, N.A. et al. Targeting key proximal drivers of type 2 inflammation in disease. Nat Rev Drug Discov 15, 35-50 (2016).









