The Western diet was a diet containing 21% fat, 50% carbohydrate, and 1.5% cholesterol, and this diet-induced NASH model developed obesity, impaired glucose tolerance, and hepatic steatosis.
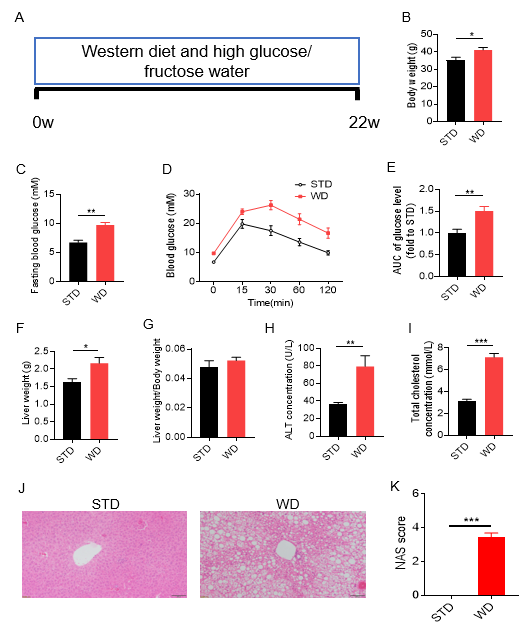
Western diet (WD) induced NASH model. Wild-type male C57BL/6J mice were randomly divided into 2 groups and given a standard diet (STD) ora Western diet (WD) diet consisting of high glucose and high fructose water, and various parameters were measured 22 weeks after feeding. (Fig. A) Schematic representation of Western diet (WD) -induced NASH mouse model; (B) body weight; (C) fasting glucose; (D) glucose tolerance test; (E) area under the curve of glucose tolerance test blood glucose level; (F) liver weight; (G) liver weight-to-body weight ratio; (H) serum ALT concentration; (I) serum total cholesterol; (J) H&E staining of liver sections; (K) NAFLD (non-alcoholic fatty liver disease) activity score (NAS). WT induced body weight gain,and increases in fasting blood glucose and glucose tolerance test blood glucose,increased liver weight, and an elevation in serum ALT and serum total cholesterol H&E staining indicates significant steatosis, and the NAS score was significantly increased. The above results indicate that WD can successfully establish a mouse model of NASH. Data are mean ± SEM, n = 8.
Efficacy Evaluation of the Anti-Glucagon Receptor (GCGR) Antibody Drug, Crotedumab, in a Mouse Model of NASH
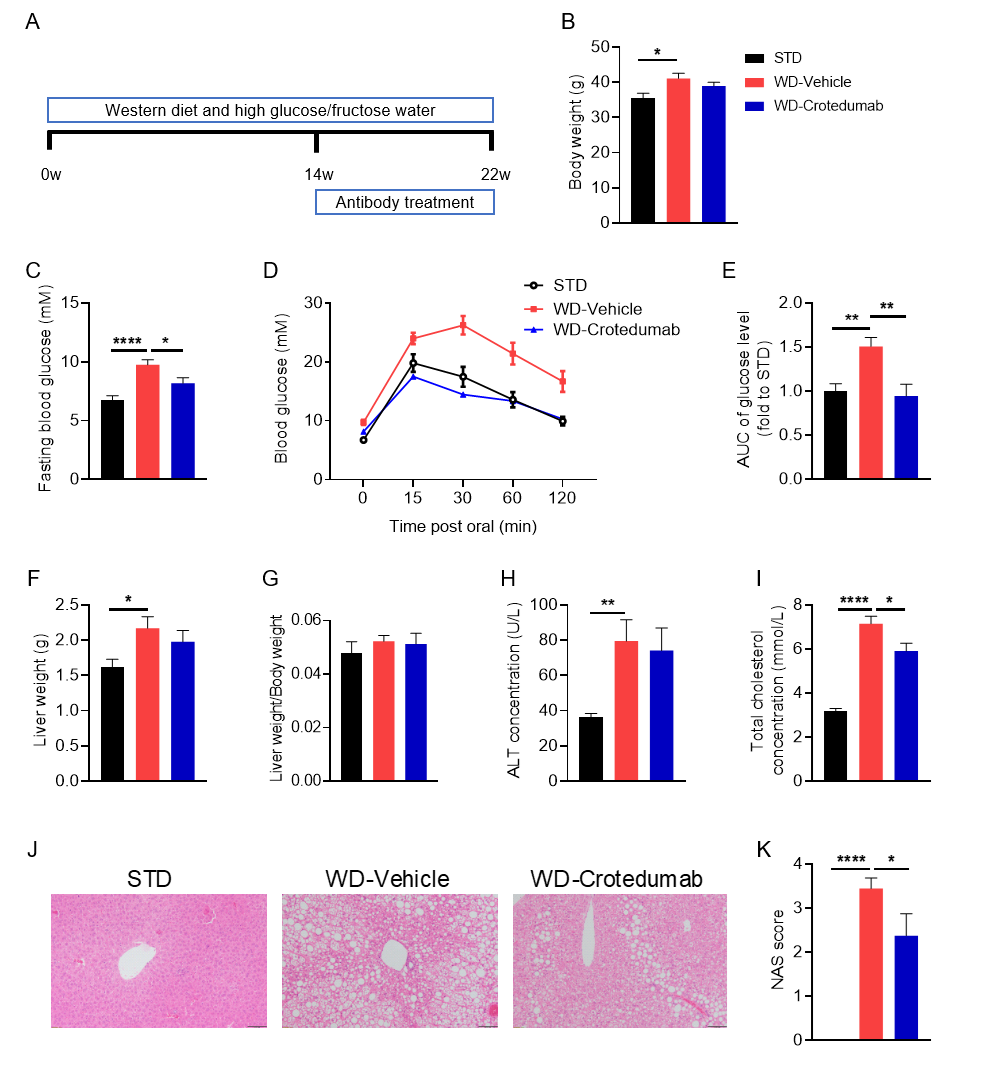
Crotedumab, a GCGR antibody, alleviated metabolic derangements in a mouse model of WD-induced NASH. ( A) Experimental schematic of NASH (non-alcoholic steatohepatitis) model establishment. Eight-week-old C57BL/6 male mice were fed a standard diet (STD) or a diet rich in cholesterol (Western diet; WD) containing 21% fat, 50% carbohydrate, and 1.5% cholesterol for 22 weeks. Blood glucose levels were analyzed and glucose tolerance tests were performed at week 13. Blood samples were collected at week 14 and analyzed for ALT and blood cholesterol, and blood samples were treated with rotedumab analog (synthesized in-house); (B) body weight; (C) fasting blood glucose; (D-E) glucose tolerance test and relative values of area under the curve; (F) liver weight; (G) ratio of liver weight to body weight; (H-I) serum ALT and total cholesterol concentrations; and (J-K) H&E staining and NAS scoring of liver tissue sections. The results indicate that drug administration significantly reduced fasting blood glucose and glucose tolerance. In addition,; liver weight and ALT were slightly decreased, serum total cholesterol concentration was decreased, and liver tissue steatosis and ballooning were relieved. The above results indicate that the application of glucagon receptor (GCGR) antibody can significantly reduce the symptoms of non-alcoholic steatohepatitis induced by Western diet. Data are mean ± SEM, n = 10.
References:
1. Hernandez-Perez, E., Leon Garcia, P.E., Lopez-Diazguerrero, N.E., Rivera-Cabrera, F. & Del Angel Benitez, E. Liver steatosis and nonalcoholic steatohepatitis: from pathogenesis to therapy. Medwave 16, e6535 (2016).
2. Tsuchida, T., et al. A simple diet- and chemical-induced murine NASH model with rapid progression of steatohepatitis, fibrosis and liver cancer. J Hepatol 69, 385-395 (2018).
3. Farrell, G., et al. Mouse Models of Nonalcoholic Steatohepatitis: Toward Optimization of Their Relevance to Human Nonalcoholic Steatohepatitis. Hepatology 69, 2241-2257 (2019).









