Asthma is a chronic airway inflammatory disease characterized by obstruction, airway hyperreactivity (AHR), and inflammation. The main clinical symptoms of asthma are shortness of breath, wheezing, cough, and increased mucus secretion after exposure to allergens. The pathogenesis of asthma is caused by a complex interplay of genetic, epigenetic, and environmental factors. Pathological changes are mediated by various cells involved in the immune response, including as airway epithelial cells, eosinophils and T lymphocytes. In particular, Th2 cells are considered to dominate hypereosinophilic asthma, through the production of by increased levels of cytokines IL-4, IL-5 and IL-13. The classical mouse model of asthma is induced by ovalbumin (OVA), in which mice are sensitized by multiple intraperitoneal injections of OVA and challenged after aerosol inhalation of OVA. In this acute asthma model, serum IgE and eosinophil levels in bronchoalveolar lavage fluid (BALF) are increased, and staining of pathological tissue sections demonstrates : increased airway mucus production and inflammatory leukocyte infiltration.
Biocytogen provides an effective OVA-induced asthma induction protocol that has been validated in different strains of mice (including C57BL/6, humanized B-hIL4/IL4RAmice, and B-hIL33 mice) for efficacy evaluation of novel asthma therapeutics.
Experimental Animals:C57BL/6, BALB/c, 5-6 weeks old, female
Modeling reagent:OVA + Al(OH)3
Modeling method:Sensitization phase: OVA + Al(OH)3 was injected intraperitoneally on days 0, 7, and 14;
challenge phase: mice were nebulized with 2% OVA for 30 min daily on days 21 – 25.
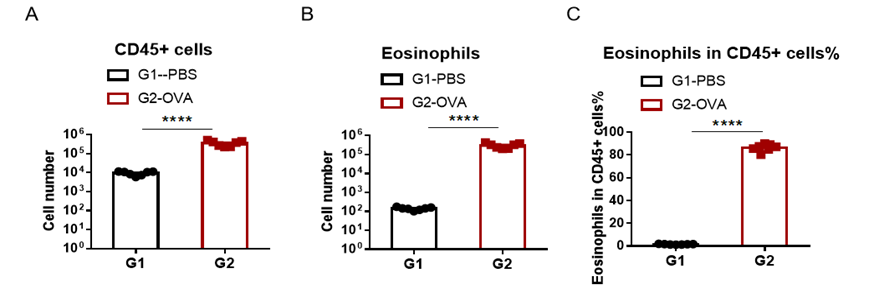
Immune Cell Infiltration in Bronchoalveolar Lavage Fluid (BALF) of Asthmatic Mice
IgE Induction in Serum of Asthmatic Mice
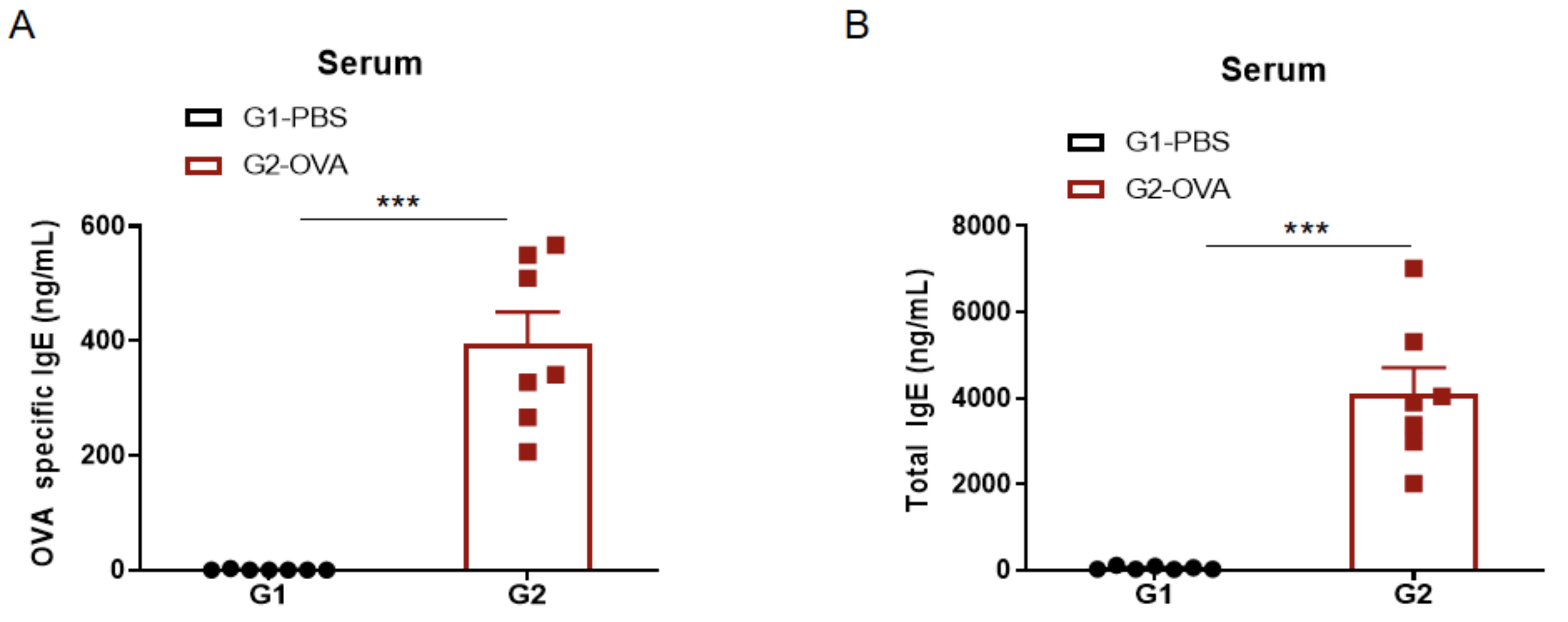
Increased IgE levels in serum of OVA-induced mice compared with controls. Serum was isolated at the end of the experiment and concentrations of OVA-specific IgE (A) and serum total IgE (B) were measured using ELISA.
Airway Histology in Asthmatic Mouse Model
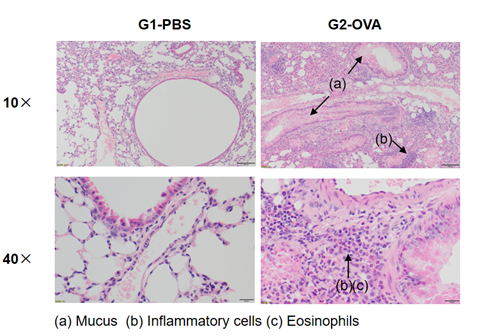
H&E staining in the lungs of asthmatic mice. In contrast to the G1 untreated group, the OVA-treated G2 model animals showed asthma-related pathological changes as demonstrated by vascular and peribronchial mixed inflammatory cell infiltration (b) and mucus (a) formation in some bronchi. The above results indicate that OVA could successfully induces asthma in wild-type C57BL/6 mice.
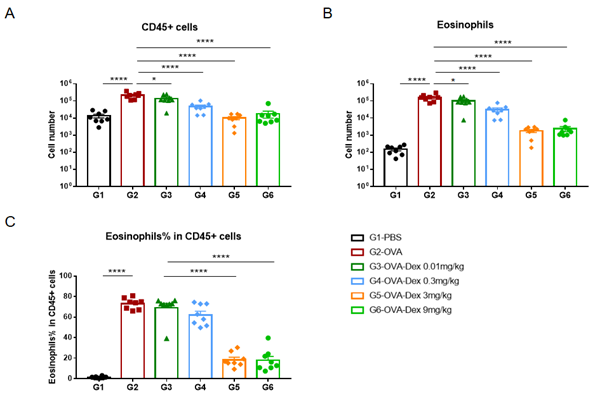
Quantification of Immune Cells in Bronchoalveolar Lavage Fluid (BALF) Of Asthmatic Mice. Asthma was induced in wild-type BALB/c mice using OVA. (A) number of CD45+ cells in BALF; (B) number of eosinophils in BALF; (C) frequency of eosinophils in the CD45+ cell population after sensitization and challenge with OVA, the white blood cell count of mice in the G2 model group was significantly increased compared with the G1 control group, and the number and proportion of eosinophils were significantly increased, suggesting that the model was successfully established. The number of CD45+ cells and eosinophils were significantly decreased in asthmatic animals treated with higher levels of dexamethasone compared with the untreated asthmatic mice (G2) group.
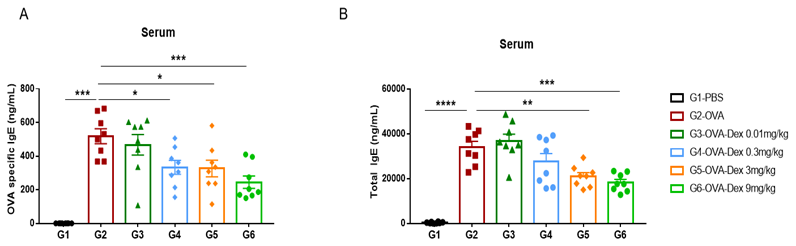
Detection of IgE levels in serum of asthmaic mice. Serum was taken at the experimental endpoint and concentrations of OVA-specific IgE (A) and total IgE (B) were measured using ELISA. The serum levels of OVA-specific IgE and total IgE in the G2 model group were significantly increased compared with those in the G1 control group, suggesting that the model was successfully established. After administration of different concentrations of dexamethasone (Dex), the specific IgE and total IgE levels were significantly reduced in a dose-dependent manner compared with the G2 group.
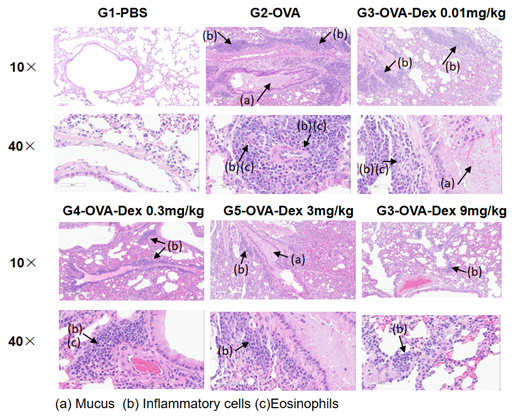
H&E staining in the lungs of asthmatic mice. The results showed that there was no inflammatory reaction in the pulmonary airway of G1 control group. Vascular and peribronchial inflammation was significantly increased and mucus secretion levels were increased in the G2 (OVA only) group, suggesting successful modeling of the disease. Administration of different concentrations of dexamethasone to groups G3, reduced inflammatory infiltration and mucus secretion. The results demonstrate that the OVA-induced mouse asthma model can be successfully used to verify the efficacy of corticosteroid immunosuppressive agents.
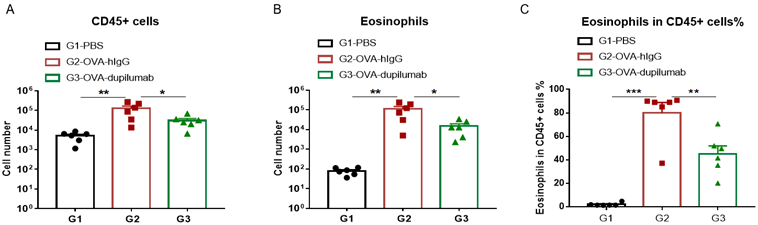
The asthma model was induced in B-hIL4/hIL4RA mice using OVA. ( A) The number of CD45 + cells in BALF. (B) The number of eosinophils in BALF. (C) The proportion of eosinophils to CD45 + cells. The results showed that after sensitization and challenge with OVA, the leukocyte infiltration of mice in G2 model group was significantly increased compared with G1 control group, and their eosinophil content was significantly increased, suggesting that the model was successfully established. After administration of dupilumab (in house), the numbers of CD45 + cells and eosinophils were significantly lower compared with the G2 model group.
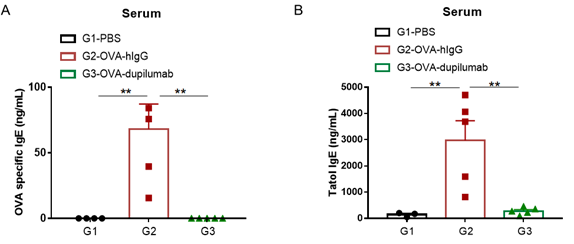
Detection of IgE levels in serum of asthmatic mouse model. Serum was taken at the end of the experiment and OVA-specific IgE and total IgE levels were measured using ELISA. (A) OVA-specific IgE levels in serum. (B) Total IgE levels in serum. The results showed that the levels of OVA-specific IgE and total IgE in G2 model group were significantly increased compared with G1 control group, suggesting successful modeling. Specific and total IgE levels were significantly lower after administration of dupilumab (in house) drug compared with the G2 modeling group.
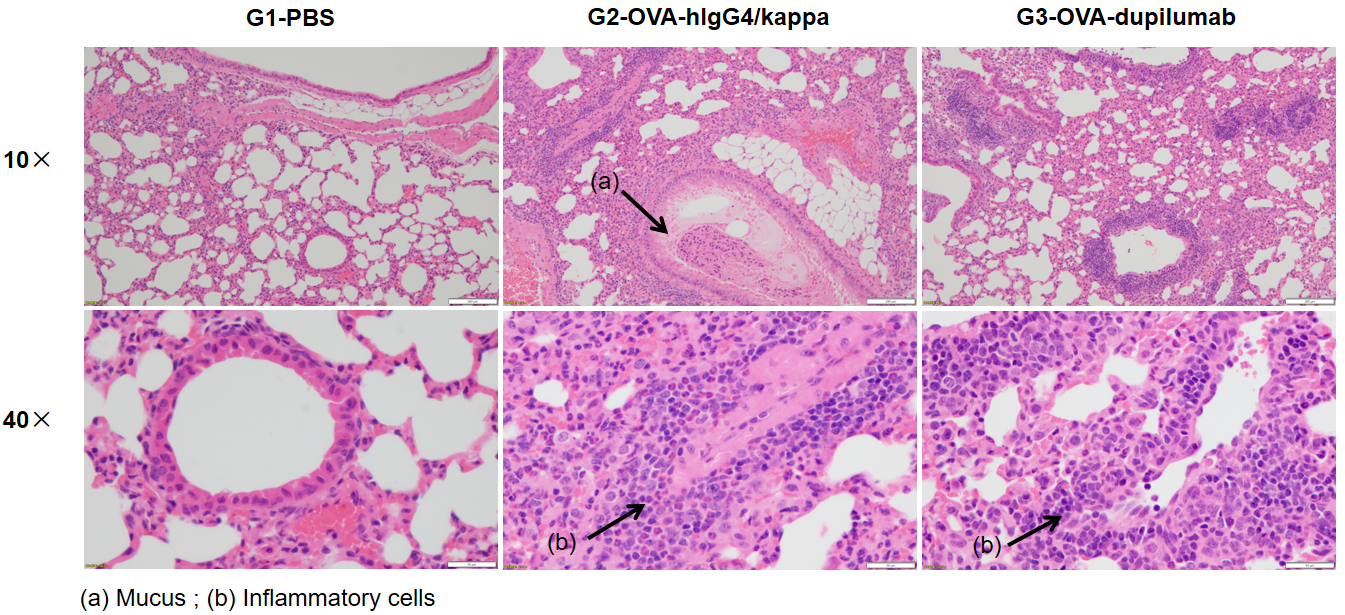
Analysis of H&E staining in the lungs of asthmatic mouse models. The results showed that there was no inflammatory cell infiltration in the pulmonary airway of G1 control group. Vascular and peribronchial inflammation (b) were significantly increased and mucus (a) secretion levels were increased in the G2 modeling group, suggesting successful modeling. G3 showed decreased inflammatory infiltration and mucus secretion in dupilumab (in house) -treated mice. The above results demonstrated that OVA could successfully establish an asthma model in B-hIL4/hIL4RA mice for efficacy evaluation.
Product list

References
1. Gandhi, N.A. et al. Targeting key proximal drivers of type 2 inflammation in disease. Nat Rev Drug Discov 15, 35-50 (2016).
2. Pelaia, G., Vatrella, A. & Maselli, R. The potential of biologics for the treatment of asthma. Nat Rev Drug Discov 11, 958-972 (2012).









