In spontaneous tumor models, mice are gene-edited to carry oncogenes or anti-oncogene mutations, allowing spontaneous tumor formation in immunocompetent mice. Compared with the tumor transplantation mice, this model exhibits genetic heterogeneity and spontaneous tumor metastasis in the spontaneous tumor tissue, which resembles the histopathological and molecular characteristics of human tumor tissue. As a result, it can better simulate the human tumor formation and development processes.
The P53 gene is most relevant to human tumors to date. P53 is an anti-oncogene, known as "the Guardian of the Genome" that regulates the cell cycle and participates in DNA damage repair. When cells are damaged beyond repair[adj], p53 may induce apoptosis to prevent Tumorigenesis. It has been shown that about half of human tumors occur in association with P53 gene mutations. Knockout or mutation of P53 may lead to spontaneous tumorigenesis in mice, which is one of the best models for studying human tumors.
Two P53 gene-associated spontaneous tumor models, B-p53 KO mice and B-p53-KO rats, are available from Biocytogen.
B-p53 KO mice
Project background
The Trp53 gene encodes tumor protein p53, which involves in diverse cellular stresses to regulate target genes that induce cell cycle arrest, apoptosis, senescence, DNA repair, or changes in metabolism. P53 protein is expressed at low level in normal cells and at a high level in a variety of transformed cell lines. Mice with homozygous p53 inactivation are developmentally normal but are susceptible to spontaneous tumors.
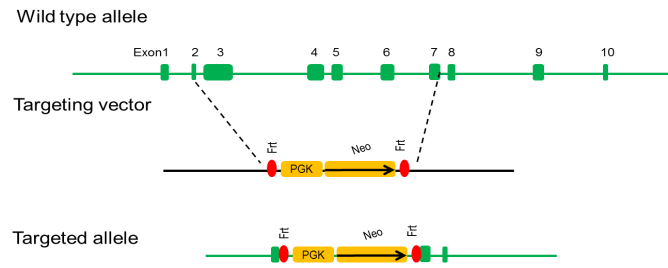
Targeting strategy
B-p53-KO rats
Project background










