Tumor-associated antigens (TAAs) are antigenic molecules that are unique to or highly expressed on tumor cells and absent or lowly expressed on healthy cells. They are involved in the proliferation, differentiation, and migration of tumor cells, thus becoming potentially effective molecular targets of anti-tumor drugs. TAA-related drugs are able to kill tumor cells by mediating antibody-dependent cell-mediated cytotoxicity (ADCC)/complement-dependent cytotoxicitytest (CDC) and activating immune responses. The efficacy of these drugs can be evaluated via subcutaneous/in situ inoculation of TAA-humanized murine-derived cell lines in wild-type mice.
To meet the current market demand for the development of TAA-related drugs, Biocytogen has developed a set of humanized cell lines for efficacy evaluation.
* Some TAA targets are expressed in healthy tissues or cells and perform important physiological functions, and drugs directing against such targets may have serious toxic side effects. Therefore, Biocytogen provides corresponding humanized animal models for drug safety evaluation.
|
310705 |
B-hB7-H3 EL4 |
310708 |
B-hEPCAM MC38 |
|
310704 |
B-hB7-H3 MC38 |
310825 |
B-hFAP MC38 |
|
310720 |
B-hBCMA MC38 |
310724 |
B-hHER2 EL4 |
|
310712 |
B-hCD19 EL4 |
311147 |
B-hHLA-E MC38 PLUS |
|
310706 |
B-hCD19 MC38 |
310693 |
B-hHVEM MC38 |
|
310717 |
B-hCD20 EL4 |
310815 |
B-hLILRB4 MC38 |
|
310716 |
B-hCD20 MC38 |
311600 |
B-hMERTK MC38 |
|
310976 |
B-hCD37 MC38 |
320714 |
B-hPD-L1 plus/hCD47 MC38 |
|
310695 |
B-hCD47 MC38 |
320722 |
B-hPD-L1 plus/hCEA MC38 |
|
310690 |
B-hCEACAM1 MC38 |
310703 |
B-hPD-L2 MC38 |
|
310791 |
B-hCLDN18.2 A549 |
310807 |
B-hROR1 MC38 |
|
310715 |
B-hCLDN18.2 MC38 |
311082 |
B-hTF MC38 |
|
310824 |
B-hCXCR2 MC38 |
311068 |
B-hTPBG MC38 |
|
310896 |
B-hCXCR4 MC38 |
310702 |
B-hCD47 CT26.WT |
|
310713 |
B-hEPCAM EL4 |
310696 |
B-hCD47 MC38 plus |
Drug target-humanized mice are animal models thatmurine protein drug target is knock out, and the human counterpart protein knocked in. This model overcomes species specificity issues of syngeneic models, which can only be used for surrogate antibodies. Also, it provides more stable results than the model of human immune system reconstitution in immunodeficient mice. This model can be used to evaluate immunotherapy efficacy because of its health immune system .
Biocytogen has created a number of humanized mouse models with single, dual, or multiple targets in response to the current market need for innovative drug development. The company has also developed humanized tumor cell lines according to the potential drug action mechanisms, so as to provide a more comprehensive and effective disease model for drug efficacy evaluation.
The humanized immune checkpoint, humanized cytokine, humanized GPCR, and other humanized mice models are available from Biocytogen. For details, see Humanized Animal Models .
Gene description
TNFRSF9 (Tumor necrosis factor receptor super family, member 9), also called CD137 and 4-1BB, is a co-stimulatory molecule and is mainly expressed on the surface of T, B, NK and mononuclear cells. CD137 is activated by its ligand CD137L or activating anti-CD137 antibodies enhance tumor rejection because it is upregulated on T cells following activation and its engagement increases T cell proliferation and pro-inflammatory cytokine production. The clinical development of 4-1BB targeting therapy was slow due to the toxicity associated with overt immune activation. Fortunately, new therapeutic modalities using 4-1BB targeting aptamers as well as therapeutic combinations with other immuno-modulatory and traditional anti-cancer treatments have revived excitement for the use of 4-1BB agonists in the clinical.
Protein expression analysis
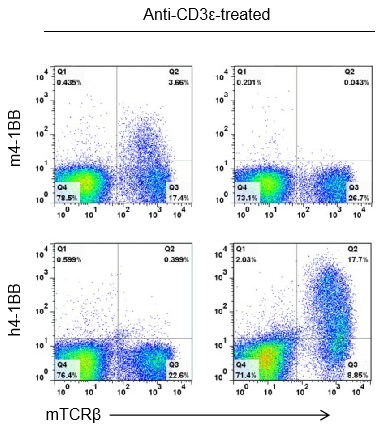

Strain specific 4-1BB protein expression analysis in wild-type (+/+) or homozygous B-h4-1BB (H/H) mice by flow cytometry. Splenocytes were collected from +/+ and homozygous mice stimulated with anti-CD3ε in vivo, and analyzed by flow cytometry with species-specific anti-4-1BB antibodies. Mouse 4-1BB was detectable in +/+ mice, while human 4-1BB was exclusively detectable in B-h4-1BB H/H mice.
High dose of urelumab resulted in liver toxicity in B-h4-1BB mice
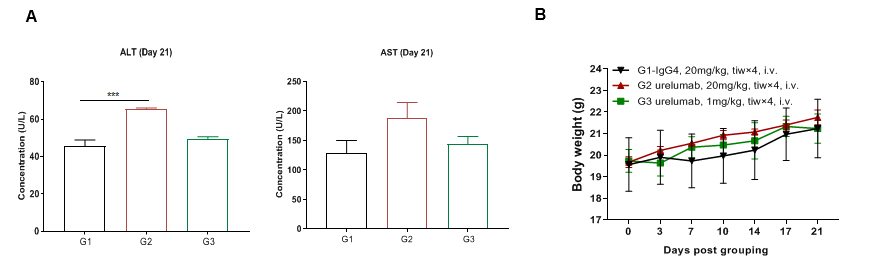
High dose of urelumab therapy resulted in increased lymphocyte infiltration in liver in B-h4-1BB mice
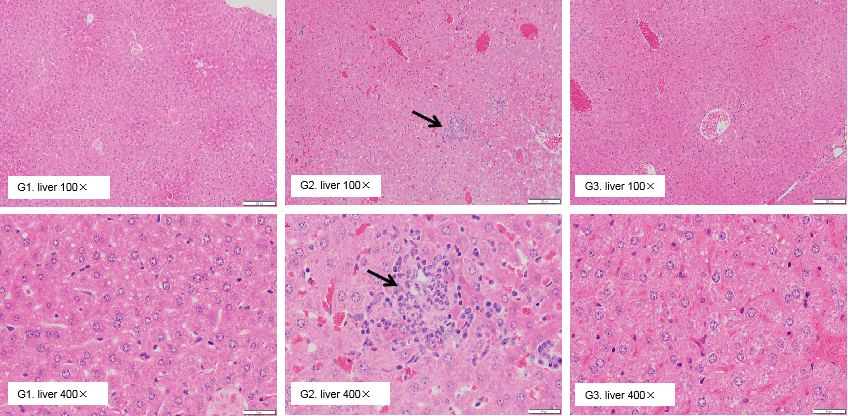
Pathological analysis of liver toxicity. Chronic inflammation (arrow) was observed in the liver of B-h4-1BB mice which were treated with high dose (20mg/kg) of urelumab (in house) for 21 days. While there were no microscopic changes in groups G1 and G3 which respectively treated with low dose (1mg/kg) of urelumab (in house) and high dose (20mg/kg) of Isotype.
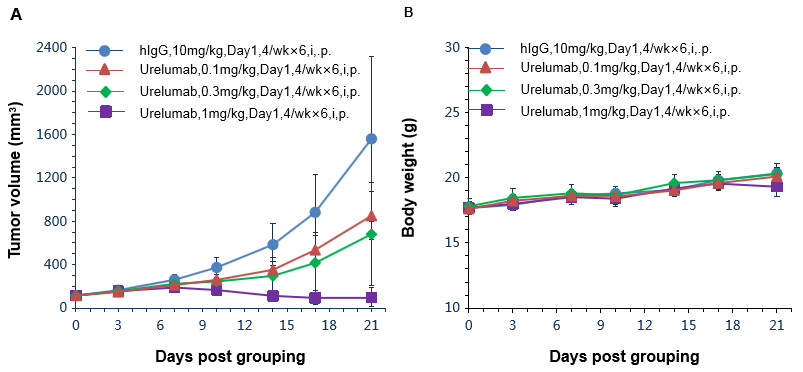
Antitumor activity of anti-human 4-1BB antibodies in B-h4-1BB mice. (A) Anti-human 4-1BB antibodies inhibited MC38 tumor growth in B-h4-1BB mice. Murine colon cancer MC38 cells were subcutaneously implanted into homozygous B-h4-1BB mice (female, 6-7 week-old, n=6). Mice were grouped when tumor volume reached approximately 100 mm3, at which time they were treated with anti-human 4-1BB antibody urelumab (in house) with doses and schedules indicated in panel. (B) Body weight changes during treatment. As shown in panel A, anti-human 4-1BB antibodies were efficacious in controlling tumor growth in B-h4-1BB mice, demonstrating that the B-h4-1BB mice provide a powerful preclinical model for in vivo evaluation of anti-human 4-1BB antibodies. Values are expressed as mean ± SEM.









