
C57BL/6-Pdcd1tm1(PDCD1)BcgenCd274tm1(CD274)BcgenVegfatm1(VEGFA)Bcgen/Bcgen • 112706

| Product name | B-hPD-1/hPD-L1/hVEGFA mice |
|---|---|
| Catalog number | 112706 |
| Strain name | C57BL/6-Pdcd1tm1(PDCD1)BcgenCd274tm1(CD274)BcgenVegfatm1(VEGFA)Bcgen/Bcgen |
| Strain background | C57BL/6 |
| NCBI gene ID | 5133,29126,7422 (Human) |
| Aliases | PD1; PD-1; CD279; SLEB2; hPD-1; hPD-l; hSLE1; ADMIO4; AIMTBS; B7-H; B7H1; PDL1; PD-L1; ADMIO5; hPD-L1; PDCD1L1; PDCD1LG1; VPF; VEGF; MVCD1; L-VEGF |
Key Advantages
Gene targeting strategy for B-hPD-1/hPD-L1/hVEGFA mice.
The exon 2 of mouse Pdcd1 gene that encodes the IgV domain was replaced by human PDCD1 exon 2 in B-hPD-1/hPD-L1/hVEGFA mice. The exon 3 of mouse Cd274 gene that encodes the IgV domain was replaced by human CD274 exon 3 in B-hPD-1/hPD-L1/hVEGFA mice. The exons 1-8 of mouse Vegfa gene that encode the full-length protein were replaced by human VEGFA exons 1-8 in B-hPD-1/hPD-L1/hVEGFA mice.
The B-hPD-1/hPD-L1/hVEGFA three knock-in model, was developed by breeding the B-hPD-1 mice, the B-hPD-L1 mice and the B-hVEGFA mice.
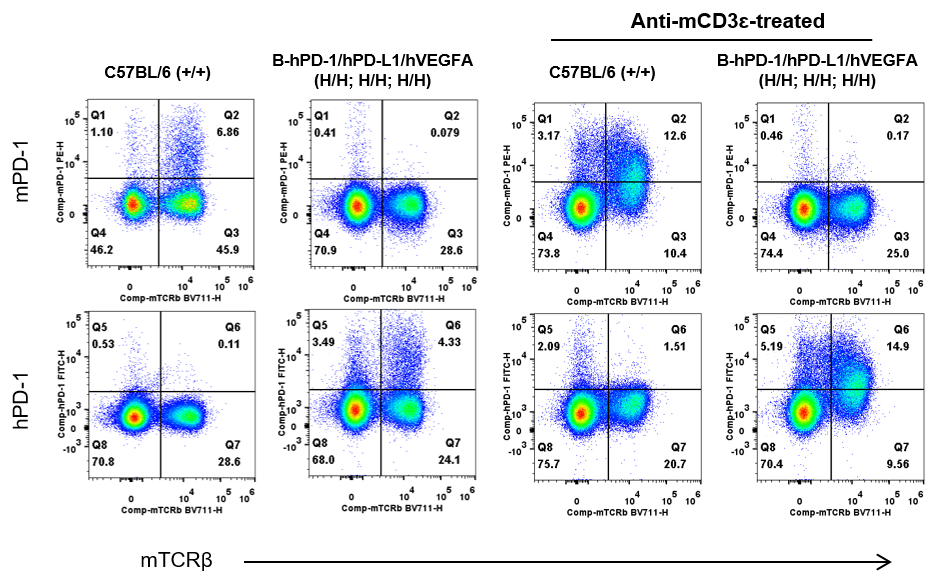
Strain specific PD-1 expression analysis in wild-type C57BL/6 and homozygous B-hPD-1/hPD-L1/hVEGFA mice by flow cytometry. Splenocytes were collected from wild-type C57BL/6 mice (+/+) and homozygous B-hPD-1/hPD-L1/hVEGFA mice (H/H) after stimulated with anti-mouse CD3ε antibody (7.5 μg, i.p.) in vivo for 24 hrs (female, 6-week-old, n=1) or not. Protein expression was analyzed with anti-mouse PD-1 antibody (Biolegend, 109104) and anti-human PD-1 antibody (Biolegend, 329904) by flow cytometry. Mouse PD-1 were detectable in wild-type C57BL/6 mice. Human PD-1 were exclusively detectable in homozygous B-hPD-1/hPD-L1/hVEGFA mice but not in wild-type mice.
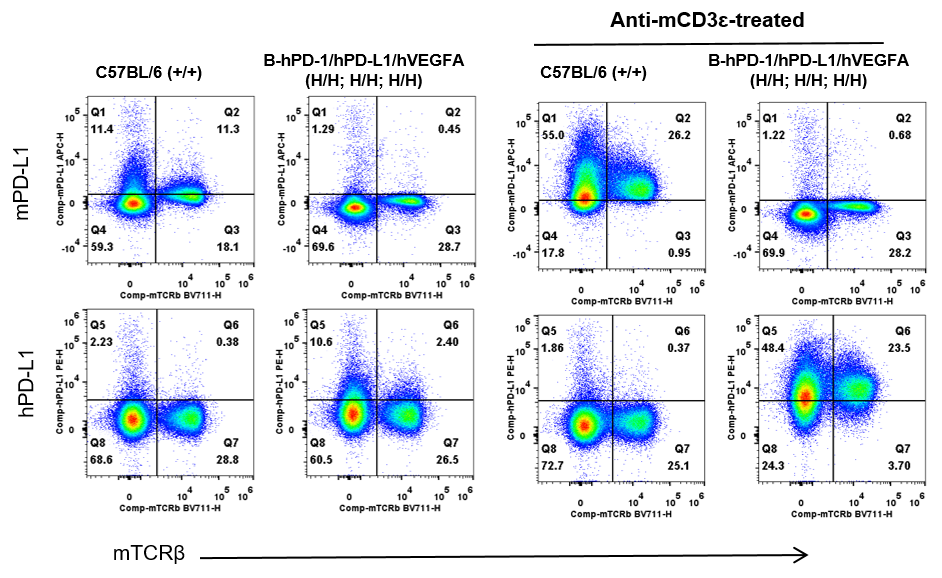
Strain specific PD-L1 expression analysis in wild-type C57BL/6 and homozygous B-hPD-1/hPD-L1/hVEGFA mice by flow cytometry. Splenocytes were collected from wild-type C57BL/6 mice (+/+) and homozygous B-hPD-1/hPD-L1/hVEGFA mice (H/H) after stimulated with anti-mouse CD3ε antibody (7.5 μg, i.p.) in vivo for 24 hrs (female, 6-week-old, n=1) or not. Protein expression was analyzed with anti-mouse PD-L1 antibody (Biolegend, 124312) and anti-human PD-L1 antibody (Biolegend, 329706) by flow cytometry. Mouse PD-L1 were detectable in wild-type C57BL/6 mice. Human PD-L1 were exclusively detectable in homozygous B-hPD-1/hPD-L1/hVEGFA mice but not in wild-type mice.
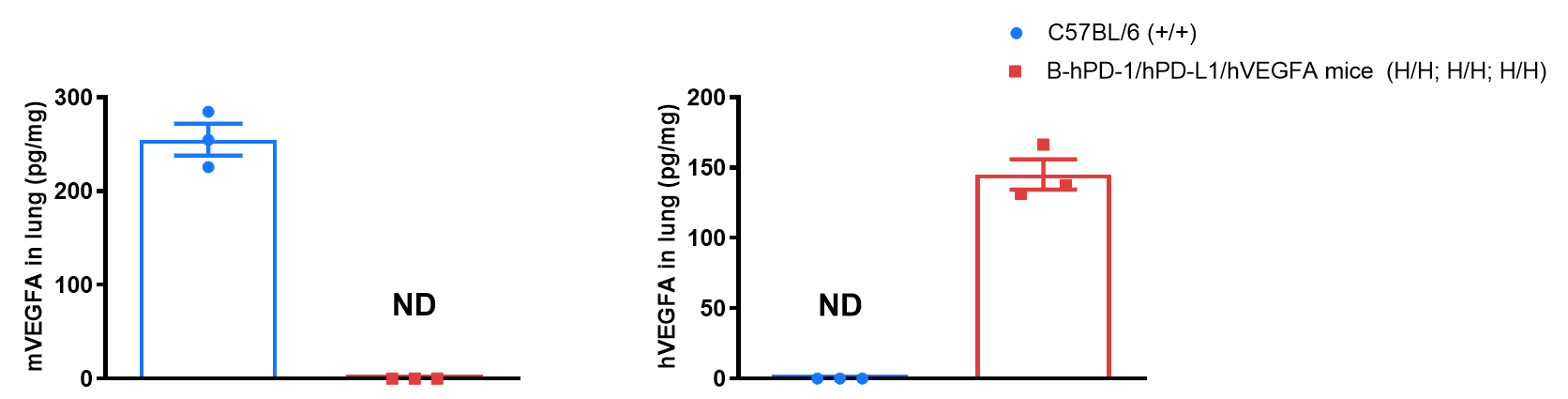
Strain specific VEGFA expression analysis in wild-type C57BL/6 mice and homozygous B-hPD-1/hPD-L1/hVEGFA mice by ELISA. Lung homogenates were collected from wild-type C57BL/6 mice (+/+) and homozygous B-hPD-1/hPD-L1/hVEGFA mice (H/H; H/H; H/H). Expression level of mouse and human VEGFA were analyzed by ELISA (anti-mouse VEGFA antibody: R&D, MMV00; anti-human VEGFA antibody: R&D, DVE00). Mouse VEGFA was detectable in wild-type mice. Human VEGFA was exclusively detectable in homozygous B-hPD-1/hPD-L1/hVEGFA mice but not in wild-type mice.
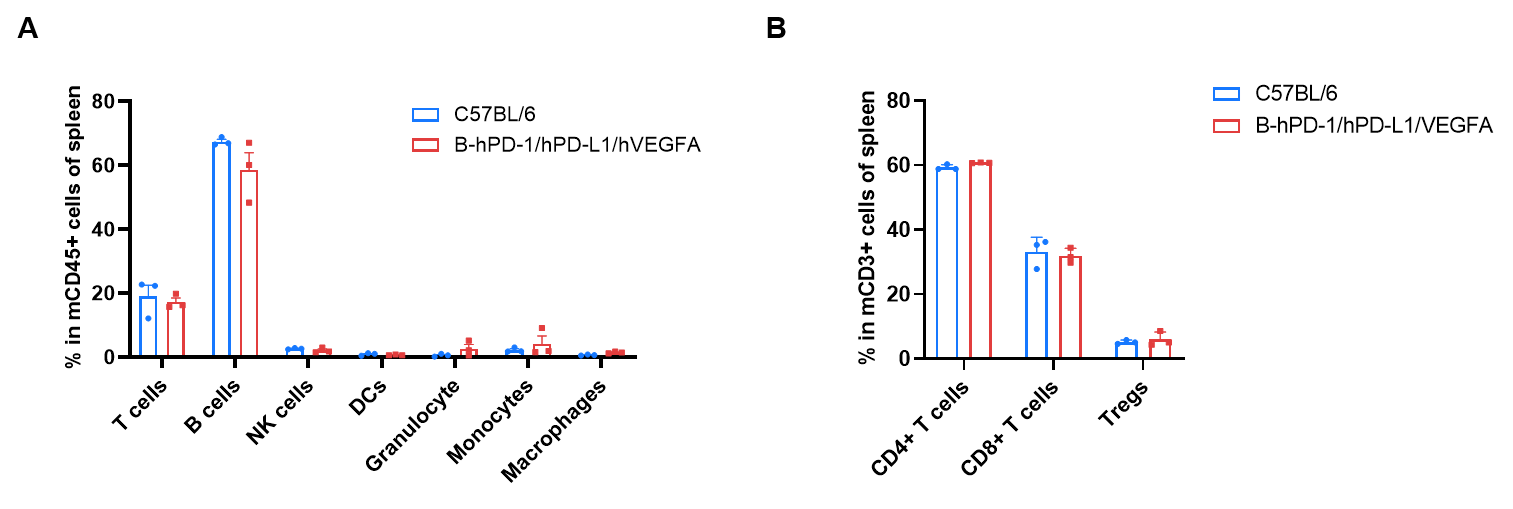
Frequency of leukocyte subpopulations in spleen by flow cytometry. Splenocytes were isolated from wild-type C57BL/6 mice and homozygous B-hPD-1/hPD-L1/hVEGFA mice (n=3, 10-week-old). A. Flow cytometry analysis of the splenocytes was performed to assess the frequency of leukocyte subpopulations. B. Frequency of T cell subpopulations. Percentages of T cells, B cells, NK cells, dendritic cells, granulocytes, monocytes, macrophages, CD4+ T cells, CD8+ T cells and Tregs in B-hPD-1/hPD-L1/hVEGFA mice were similar to those in C57BL/6 mice, demonstrating that humanization of PD-1, PD-L1 and VEGFA does not change the frequency or distribution of these cell types in spleen. Values are expressed as mean ± SEM. Significance was determined by two-way ANOVA test. *P < 0.05, **P < 0.01, ***p < 0.001.
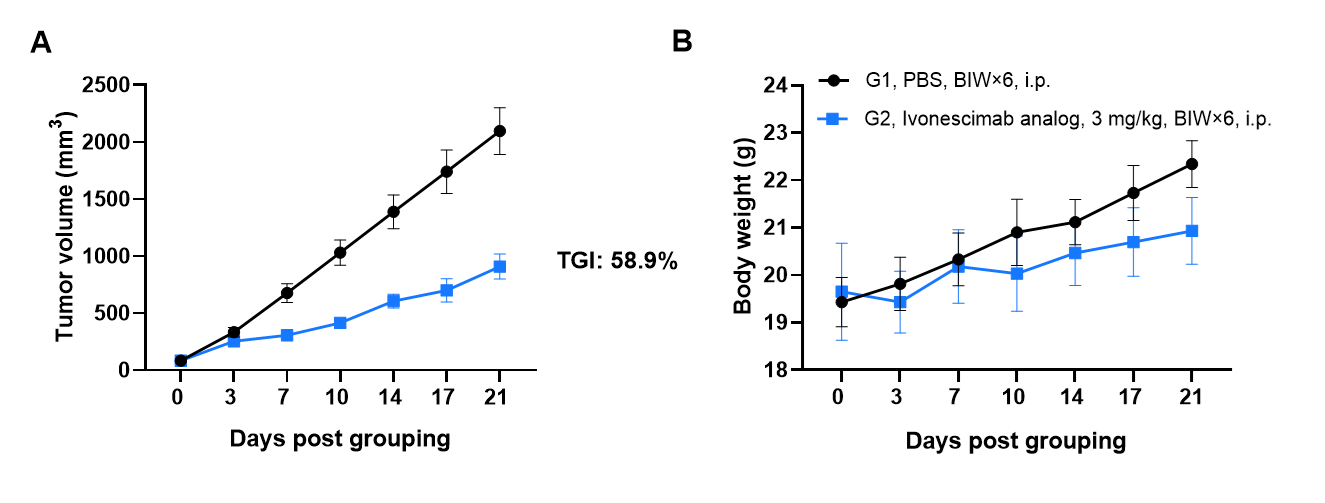
Antitumor activity of Ivonescimab analog (in-house) in B-hPD-1/hPD-L1/hVEGFA mice. Murine colon cancer B-hVEGFA MC38 cells were subcutaneously implanted into homozygous B-hPD-1/hPD-L1/hVEGFA mice (female, 8-week-old, n=6). Mice were grouped when tumor volume reached approximately 70-90 mm3, at which time they were intraperitoneally injected with anti-PD-1/VEGFA bispecific antibody indicated in panel. As shown in panel A, Ivonescimab was efficacious in controlling tumor growth in B-hPD-1/hPD-L1/hVEGFA mice. Values are expressed as mean ± SEM.
The overage of this tumor model is 50%.
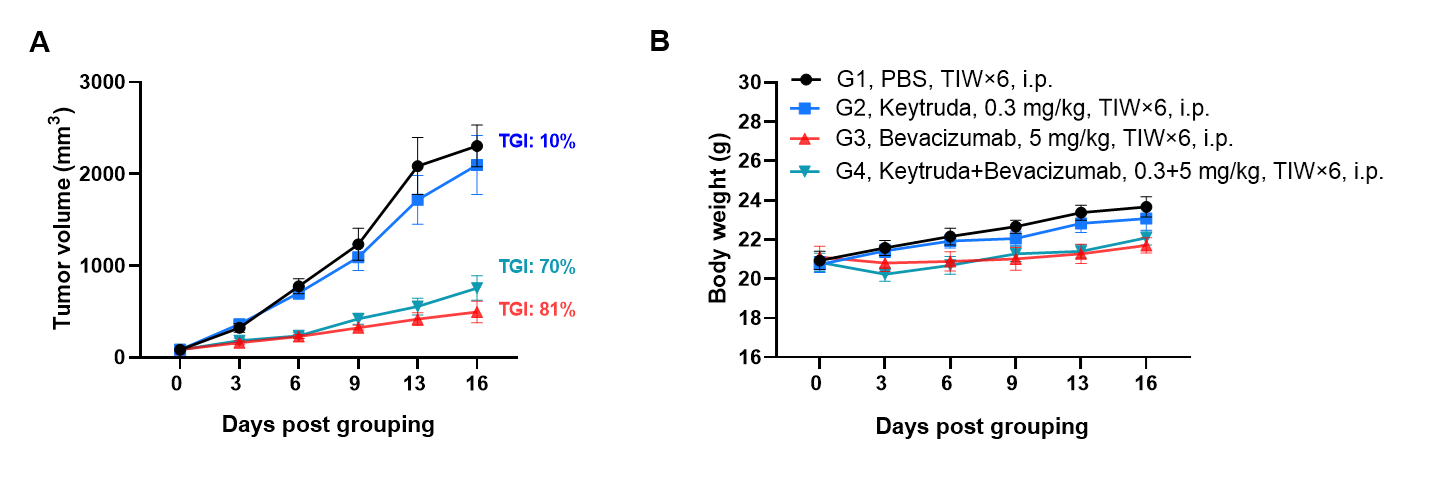
Antitumor activity of bevacizumab and Keytruda (pembrolizumab) was assessed in PD-1/PD-L1/VEGFA humanized mice implanted with murine colon cancer B-hVEGFA MC38 cells (female, 8-week-old, n=6). When tumors reached 70–90 mm³, mice were assigned to treatment groups and intraperitoneally injected with anti-PD-1 and/or anti-VEGFA antibodies. Bevacizumab monotherapy effectively controlled tumor growth in the humanized model. However, the combination of bevacizumab with Keytruda did not yield enhanced efficacy compared to monotherapy.Values are presented as mean ± SEM.
The overage of this tumor model is 40%.
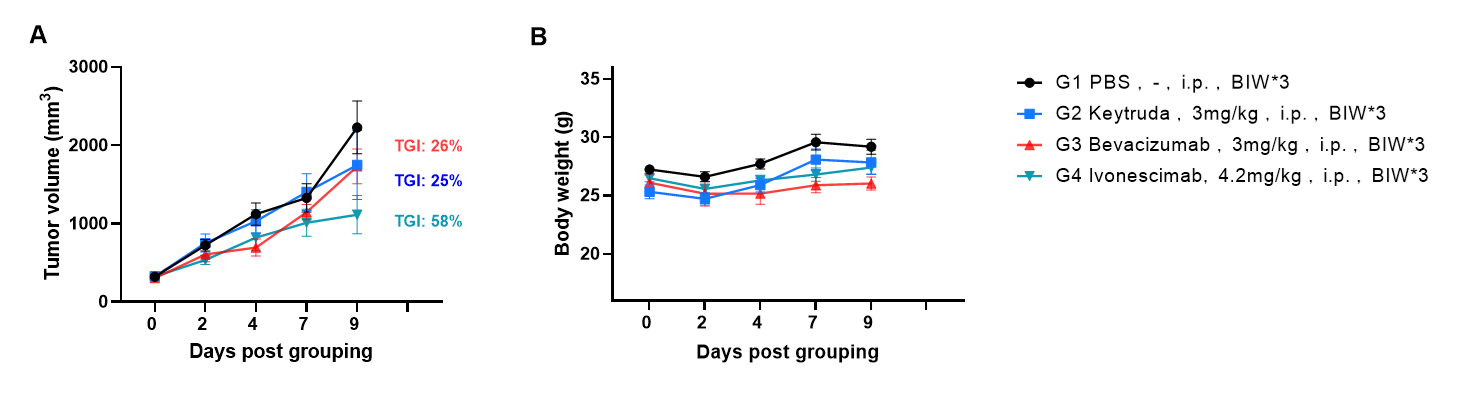
Antitumor activity of Ivonescimab (also known as AK112) in B-hPD-1/hPD-L1/hVEGFA mice. Murine colon cancer B-hVEGFA/hPD-L1 plus MC38 cells were subcutaneously implanted into homozygous B-hPD-1/hPD-L1/hVEGFA mice (male, 8-week-old, n=6). Mice were grouped when tumor volume reached approximately 300 mm3, at which time they were intraperitoneally injected with Ivonescimab indicated in panel. As shown in panel A, Ivonescimab was efficacious in controlling tumor growth in B-hPD-1/hPD-L1/hVEGFA mice. Values are expressed as mean ± SEM.
The overage of this tumor model is 60%.
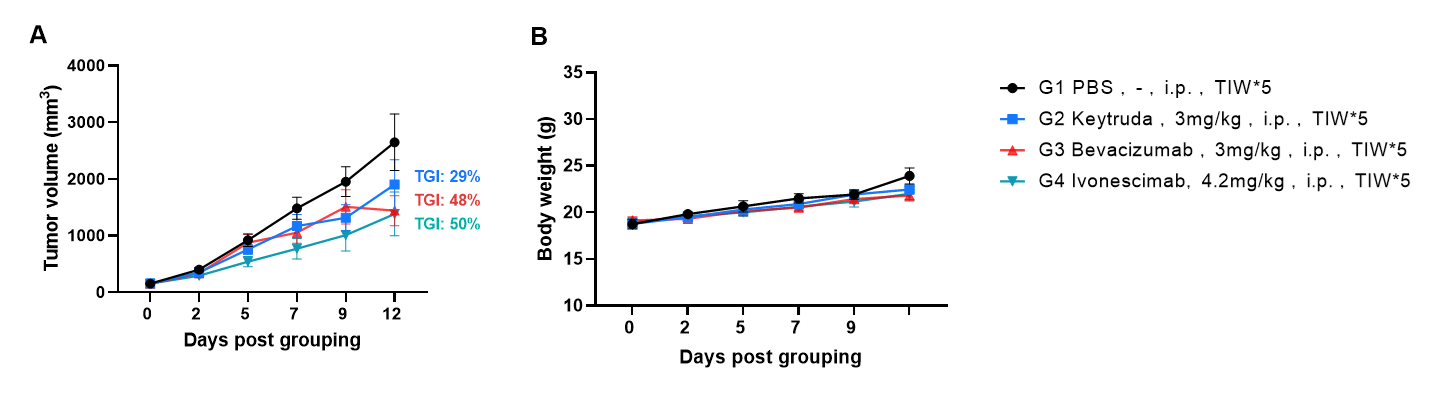
Antitumor activity of Ivonescimab (also known as AK112) in B-hPD-1/hPD-L1/hVEGFA mice. Murine melanoma cells B-hVEGFA/hPD-L1 B16-F10 were subcutaneously implanted into homozygous B-hPD-1/hPD-L1/hVEGFA mice (female, 8-week-old, n=6). Mice were grouped when tumor volume reached approximately 150 mm3, at which time they were intraperitoneally injected with Ivonescimab indicated in panel. As shown in panel A, Ivonescimab was efficacious in controlling tumor growth in B-hPD-1/hPD-L1/hVEGFA mice. Values are expressed as mean ± SEM.
The overage of this tumor model is 60%. Note: one mouse dead in G3 on day12.
Q1. What are B-hPD-1/hPD-L1/hVEGFA mice mainly used for?
B-hPD-1/hPD-L1/hVEGFA mice are triple humanized PD-1/PD-L1/VEGFA mice designed for in vivo efficacy studies of human-specific PD-1/PD-L1 checkpoint inhibitors, anti-VEGFA antibodies and anti-PD-1/VEGF bispecific antibodies, including ivonescimab and bevacizumab/Keytruda combinations in B-hVEGFA MC38 syngeneic tumor models.
Q2. How do these humanized PD-1/PD-L1/VEGFA mice differ from single humanized PD-1 mice?
Compared with single-humanized PD-1 mice, this triple-humanized PD-1/PD-L1/VEGFA model simultaneously expresses human PD-1, PD-L1, and VEGFA. It better recapitulates the clinical setting in which anti–PD-1/PD-L1 and anti-VEGF/VEGFA agents act on multiple human targets, making it more predictive for combination therapies and bispecific antibody development.
Q3. Can I use B-hPD-1/hPD-L1/hVEGFA mice for ivonescimab (AK112) preclinical studies?
Yes. Ivonescimab (AK112) has been tested in B-hPD-1/hPD-L1/hVEGFA mice bearing B-hVEGFA/hPD-L1 MC38 tumors, where it showed significant antitumor activity, demonstrating that this model is suitable for ivonescimab and other anti-PD-1/VEGF bispecific antibody evaluation.
Q4. What tumor models are recommended with B-hPD-1/hPD-L1/hVEGFA mice?
B-hVEGFA MC38 and B-hVEGFA/hPD-L1 MC38 tumor models are recommended, as they express human VEGFA and human PD-L1, ensuring fully human ligand–receptor engagement with human PD-1/PD-L1/VEGFA in the host mice.