
C57BL/6-Tnfrsf9tm1(TNFRSF9)Bcgen/Bcgen • 110004
4-1BB (CD137, TNFRSF9) is a costimulatory receptor belonging to the tumor necrosis factor receptor superfamily and is primarily expressed on activated T cells and other immune cells. Engagement of 4-1BB plays a critical role in T-cell activation, proliferation, and antitumor immune responses, making it an important target in cancer immunotherapy.
In 4-1BB Humanized Mouse Model (B-h4-1BB Mice), the endogenous mouse Tnfrsf9 gene is replaced with the human TNFRSF9 gene, resulting in exclusive expression of human 4-1BB at both the mRNA and protein levels. Validation studies demonstrate that human 4-1BB is specifically expressed in homozygous B-h4-1BB mice, while mouse 4-1BB expression is absent. This model has been extensively validated for in vivo efficacy and safety assessment of anti-human 4-1BB antibodies, including agonistic antibodies and bispecific formats. Our humanized 4-1BB Mouse Model (B-h4-1BB Mice) support tumor growth inhibition studies, immune activation analysis, tumor-infiltrating lymphocyte (TIL) profiling, and hepatotoxicity risk evaluation associated with 4-1BB agonism.
Key Advantages
Validation
Application: This model is well suited for in vivo functional, efficacy, and safety studies of anti-human 4-1BB antibodies. The model also enables mechanistic investigation of 4-1BB–mediated immune responses and tumor immunology in preclinical cancer research.
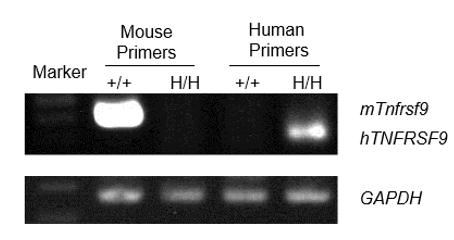
Species-specific expression of the 4-1BB gene was analyzed in wild-type C57BL/6 mice and homozygous 4-1BB humanized mice (B-h4-1BB mice) by RT-PCR. RNA extracted from splenocytes was amplified using mouse-specific and human-specific primers. Mouse 4-1BB (mTnfrsf9) mRNA was detectable in wild-type (+/+) mice but not in homozygous (H/H) 4-1BB humanized mice (B-h4-1BB mice). In contrast, human 4-1BB (hTNFRSF9) mRNA was detected exclusively in homozygous (H/H) 4-1BB humanized mice (B-h4-1BB mice) and was absent in wild-type (+/+) mice. GAPDH was used as an internal control.
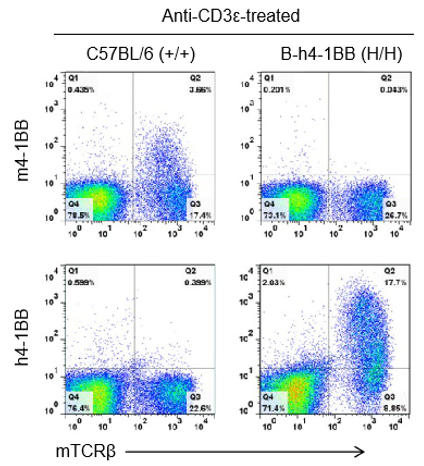
Species-specific 4-1BB protein expression analysis in wild-type C57BL/6 mice and homozygous 4-1BB humanized mice (B-h4-1BB mice) by flow cytometry. Splenocytes were collected from wild-type C57BL/6 mice (+/+) and homozygous 4-1BB humanized mice (B-h4-1BB mice, H/H) that were stimulated with anti-CD3ε in vivo, and subsequently analyzed by flow cytometry using species-specific anti–4-1BB antibodies. Mouse 4-1BB protein was detectable in C57BL/6 mice, whereas human 4-1BB protein was exclusively detected in homozygous 4-1BB humanized mice.
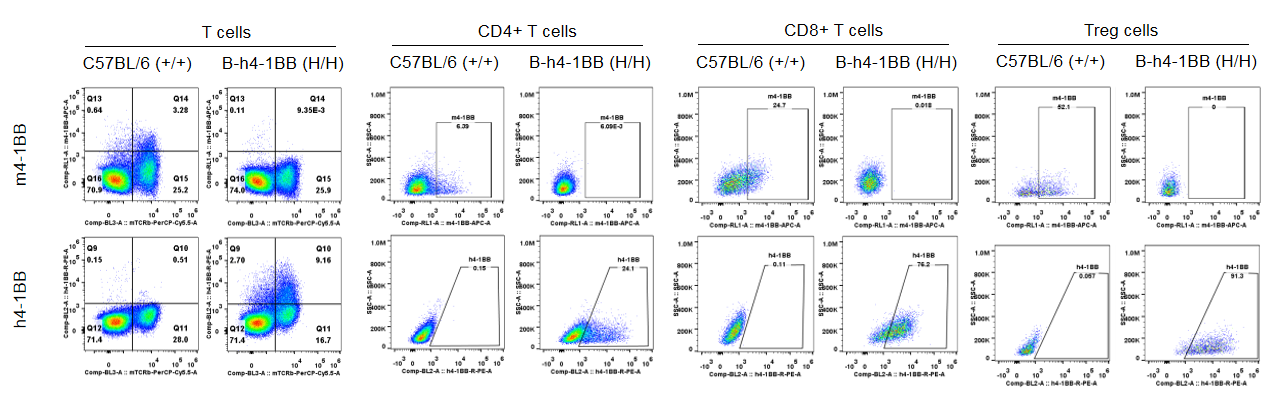
Species-specific 4-1BB protein expression in T cell subsets from wild-type C57BL/6 mice and homozygous 4-1BB humanized mice (B-h4-1BB mice) by flow cytometry. Splenocytes were collected from wild-type C57BL/6 mice (+/+) and homozygous 4-1BB humanized mice (B-h4-1BB mice, H/H) following anti-CD3ε in vivo stimulation. Cells were analyzed by flow cytometry using species-specific anti–4-1BB antibodies. Mouse 4-1BB protein was detectable in T cell subsets from C57BL/6 mice, whereas human 4-1BB protein was exclusively detected across corresponding T cell subsets in homozygous 4-1BB humanized mice.

Antitumor activity of anti-human 4-1BB antibodies in 4-1BB humanized mice (B-h4-1BB mice). Female homozygous 4-1BB humanized mice (B-h4-1BB mice; 6–7 weeks old, n = 5) were subcutaneously implanted with murine MC38 colon cancer cells and grouped when tumor volume reached approximately 100 mm³. Mice were then treated with two anti-human 4-1BB antibodies at the doses and schedules indicated. (A) Tumor growth curves showing inhibition of MC38 tumor growth following anti-human 4-1BB antibody treatment. (B) Body weight changes during the treatment period. These results demonstrate that 4-1BB humanized mice provide a robust preclinical model for in vivo evaluation of anti-human 4-1BB antibodies. Data are presented as mean ± SEM.

Antitumor activity of an anti-human 4-1BB antibody in 4-1BB humanized mice (B-h4-1BB mice). Male homozygous 4-1BB humanized mice (B-h4-1BB mice; 7–8 weeks old, n = 6) were subcutaneously implanted with murine MC38 colon cancer cells and grouped when tumor volume reached approximately 100 mm³. Mice were then treated with an anti-human 4-1BB antibody at the dose and schedule indicated. (A) Tumor growth curves showing inhibition of MC38 tumor growth following anti-human 4-1BB antibody treatment compared with vehicle control. (B) Body weight assessment during the treatment period. These results demonstrate that 4-1BB humanized mice provide a robust preclinical model for in vivo evaluation of anti-human 4-1BB antibodies. Data are presented as mean ± SEM.
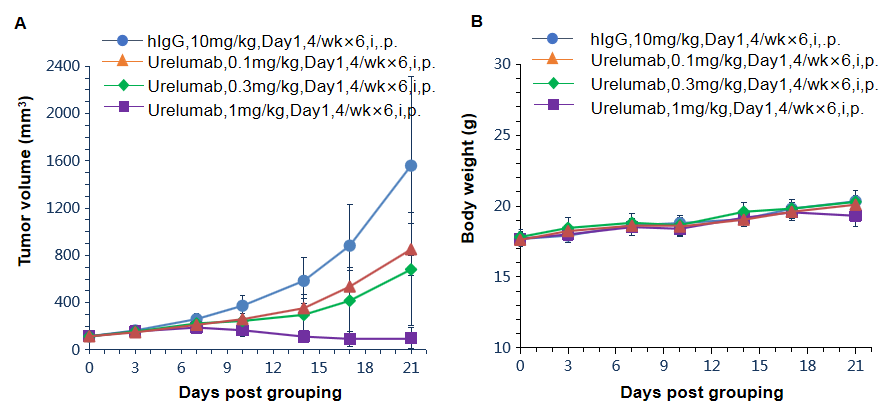
Antitumor activity of anti-human 4-1BB antibodies in 4-1BB humanized mice (B-h4-1BB mice). Female homozygous 4-1BB humanized mice (B-h4-1BB mice; 6–7 weeks old, n = 6) were subcutaneously implanted with murine MC38 colon cancer cells and grouped when tumor volume reached approximately 100 mm³. Mice were then treated with an anti-human 4-1BB antibody (Urelumab, in house) at the doses and schedules indicated. (A) Tumor growth curves showing dose-dependent inhibition of MC38 tumor growth following Urelumab treatment compared with hIgG control. (B) Body weight assessment during the treatment period. These results demonstrate that 4-1BB humanized mice provide a robust preclinical model for in vivo evaluation of anti-human 4-1BB antibodies. Data are presented as mean ± SEM.

Antitumor activity of anti-human 4-1BB antibodies in 4-1BB humanized mice (B-h4-1BB mice). Female homozygous 4-1BB humanized mice (B-h4-1BB mice; 6–7 weeks old, n = 5) were subcutaneously implanted with murine MC38 colon cancer cells and grouped when tumor volume reached approximately 100 mm³. Mice were then treated with an anti-human 4-1BB antibody (Utomilumab, in house) at 1 mg/kg or 10 mg/kg according to the dosing schedule indicated. (A) Tumor growth curves showing inhibition of MC38 tumor growth following Utomilumab treatment compared with hIgG control, with enhanced antitumor efficacy observed at the higher dose. (B) Body weight assessment during the treatment period. These results demonstrate that 4-1BB humanized mice provide a robust preclinical model for in vivo evaluation of anti-human 4-1BB antibodies. Data are presented as mean ± SEM.

Antitumor activity of anti-human 4-1BB antibodies in 4-1BB humanized mice (B-h4-1BB mice). Female homozygous 4-1BB humanized mice (B-h4-1BB mice; 6–7 weeks old, n = 8) were subcutaneously implanted with murine MC38 colon cancer cells and grouped when tumor volume reached approximately 100 mm³. Mice were then treated with two anti-human 4-1BB antibodies at the doses and schedules indicated. (A) Tumor growth curves showing marked inhibition of MC38 tumor growth following treatment with anti-human 4-1BB antibodies compared with vehicle control. (B) Body weight assessment during the treatment period. These results demonstrate that 4-1BB humanized mice provide a robust preclinical model for in vivo evaluation of anti-human 4-1BB antibodies. Data are presented as mean ± SEM.
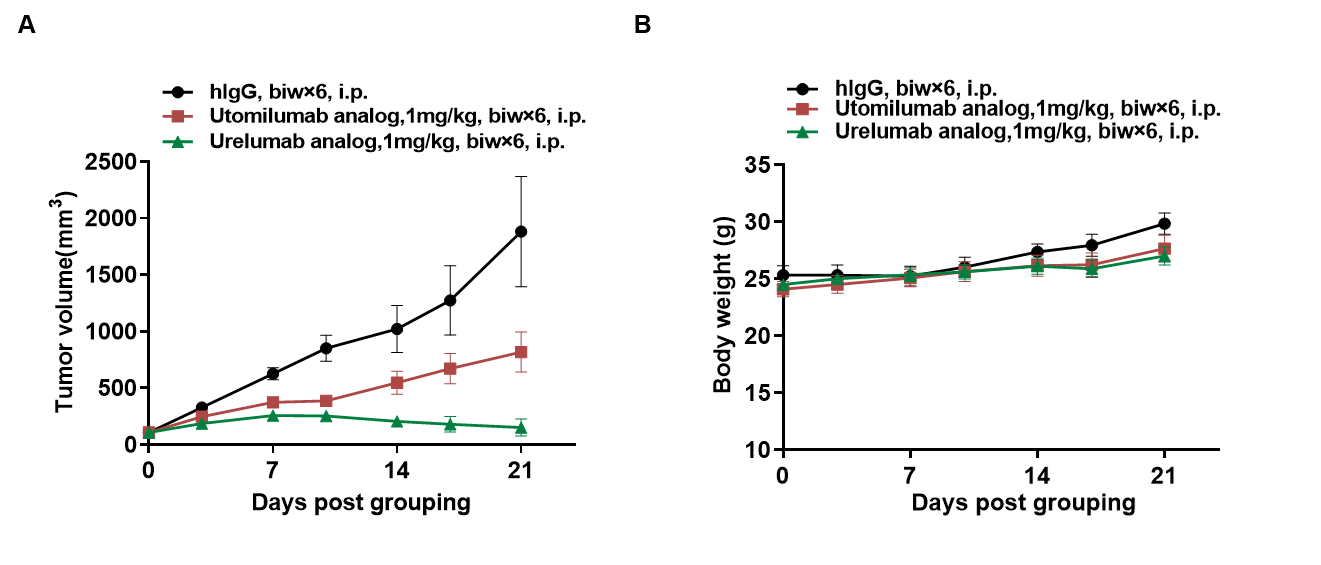
Antitumor activity of anti-human 4-1BB antibody analogs in 4-1BB humanized mice (B-h4-1BB mice). Female homozygous 4-1BB humanized mice (B-h4-1BB mice; 6–7 weeks old, n = 5) were subcutaneously implanted with murine MC38 colon cancer cells and grouped when tumor volume reached approximately 100 mm³. Mice were then treated with an Utomilumab analog or a Urelumab analog at the doses and schedules indicated. (A) Tumor growth curves showing inhibition of MC38 tumor growth following treatment with anti-human 4-1BB antibody analogs compared with hIgG control. (B) Body weight assessment during the treatment period. These results demonstrate that 4-1BB humanized mice provide a robust preclinical model for in vivo evaluation of anti-human 4-1BB antibodies. Data are presented as mean ± SEM.
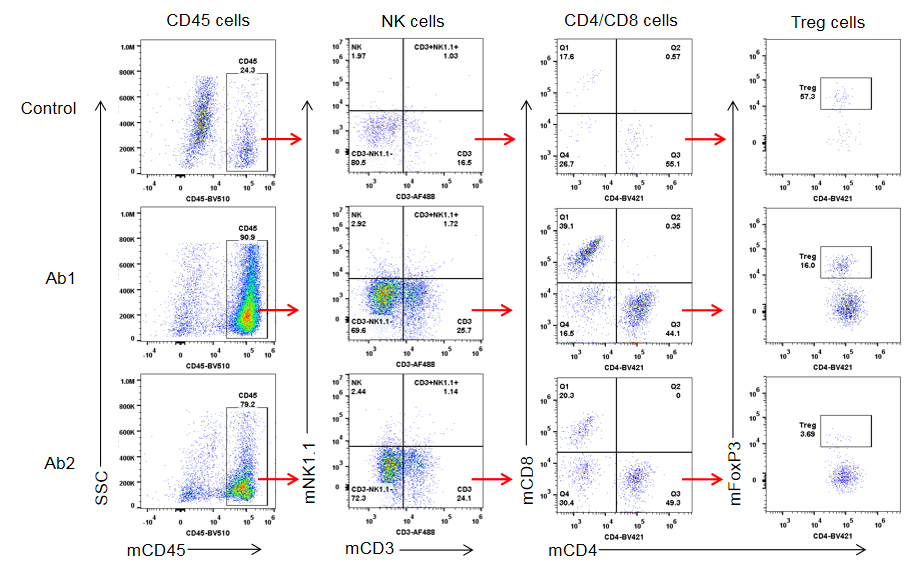
Flow cytometric analysis of tumor-infiltrating immune cell populations in 4-1BB humanized mice (B-h4-1BB Mice). Tumor tissues were collected at the experimental endpoint, dissociated into single-cell suspensions, and analyzed by flow cytometry. Tumor-infiltrating CD45⁺ leukocytes were first gated and further analyzed for NK cells, CD4⁺ and CD8⁺ T cells, and regulatory T cells (Tregs). Representative gating strategies and population distributions are shown for control-treated tumors and tumors treated with two anti-human 4-1BB antibodies (Ab1 and Ab2), illustrating treatment-associated changes in immune cell infiltration within the tumor microenvironment.
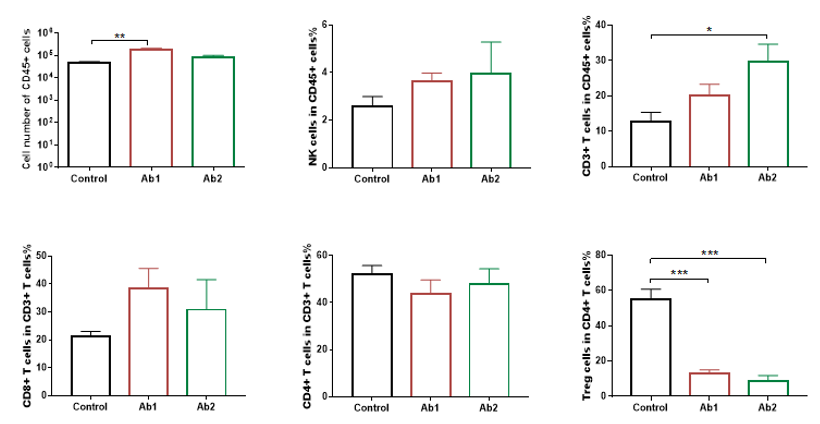
Quantitative analysis of tumor-infiltrating lymphocyte populations by flow cytometry in 4-1BB humanized mice (B-h4-1BB Mice). Tumor tissues were harvested at the experimental endpoint and analyzed by flow cytometry (n = 3). The numbers and proportions of tumor-infiltrating immune cell populations were quantified and compared among control-treated tumors and tumors treated with two anti-human 4-1BB antibodies (Ab1 and Ab2). CD45⁺ leukocytes and CD3⁺ T cells were significantly increased following anti-human 4-1BB antibody treatment, whereas regulatory T cells (Tregs) were significantly reduced in antibody-treated groups compared with the control group. NK cell and CD8⁺ T cell populations showed treatment-associated changes. Data are presented as mean ± SEM.
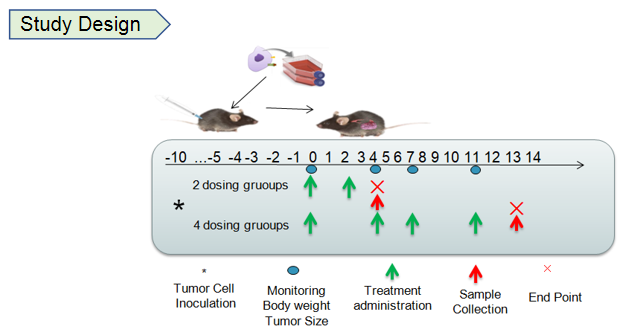

Study design and antitumor activity of anti-human 4-1BB antibodies in 4-1BB humanized mice (B-h4-1BB mice). Murine MC38 colon cancer cells were subcutaneously implanted into female homozygous 4-1BB humanized mice (B-h4-1BB mice; 6–7 weeks old, n = 5). Mice were grouped when tumor volume reached approximately 150 mm³ and treated with anti-human 4-1BB antibodies (Urelumab) according to the dosing regimens illustrated in the study design schematic. Tumor volume and body weight were monitored throughout the study period. Anti-human 4-1BB antibody treatment effectively inhibited MC38 tumor growth compared with PBS control, while body weight remained stable during treatment. These results demonstrate that 4-1BB humanized mice provide a robust preclinical model for in vivo evaluation of anti-human 4-1BB antibodies. Data are presented as mean ± SEM.
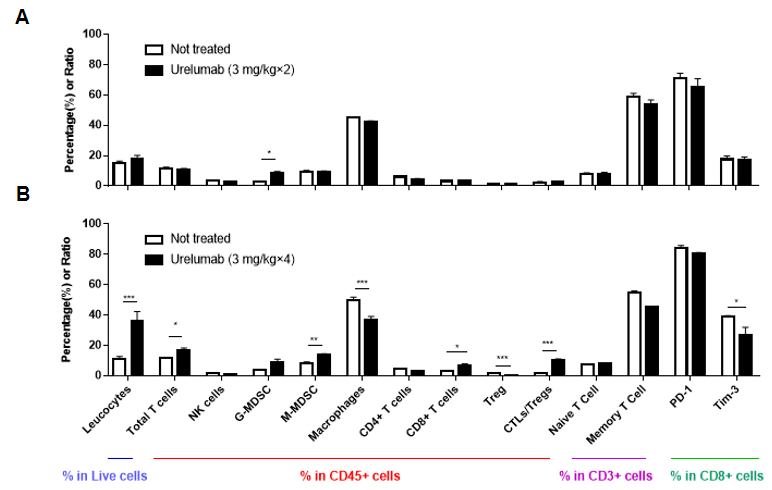
Quantitative flow cytometry analysis of tumor-infiltrating lymphocyte subsets in 4-1BB humanized mice (B-h4-1BB mice). Murine MC38 colon cancer cells were subcutaneously implanted into female homozygous 4-1BB humanized mice (B-h4-1BB mice; 6–7 weeks old, n = 5). Mice were grouped when tumor volume reached approximately 150 mm³ and treated with anti-human 4-1BB antibodies (Urelumab) according to the dosing regimens indicated. Tumors were harvested at the experimental endpoint and analyzed by flow cytometry to assess the proportions of immune cell populations within live cells, CD45⁺ leukocytes, CD3⁺ T cells, and CD8⁺ T cells. Repeated anti-human 4-1BB antibody treatment was associated with significant increases in leukocytes and total T cells, an increase in CD8⁺ T cells, and a significant reduction in regulatory T cells (Tregs) compared with untreated controls. Data are presented as mean ± SEM.
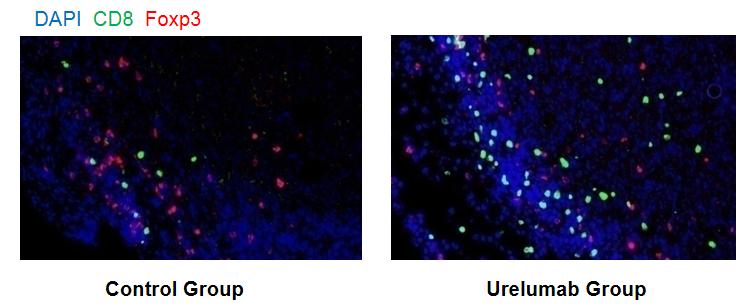
Immunofluorescence analysis of tumor immune cell infiltration in 4-1BB humanized mice (B-h4-1BB mice). Murine MC38 colon cancer cells were subcutaneously implanted into female homozygous 4-1BB humanized mice (B-h4-1BB mice; 6–7 weeks old, n = 5). Mice were grouped when tumor volume reached approximately 150 mm³ and treated with anti-human 4-1BB antibodies (Urelumab) according to the dosing regimens indicated. Tumor tissues were harvested at the experimental endpoint, paraffin-embedded, and subjected to immunofluorescence staining using antibodies specific for CD8 and Foxp3, with DAPI used for nuclear counterstaining. Representative images from control and Urelumab-treated groups are shown. Compared with the control group, the Urelumab-treated group exhibited increased infiltration of CD8⁺ T cells (green) and a reduction in Foxp3⁺ regulatory T cells (red) within the tumor microenvironment, consistent with treatment-associated modulation of intratumoral immune cell composition.

High-dose urelumab analog caused a decline in liver function in 4-1BB humanized mice (B-h4-1BB mice). 4-1BB humanized mice were divided into three treatment groups and administered hIgG4 isotype control or urelumab analogs (in house) at different doses according to the dosing regimen indicated. Blood samples were collected on days 14 and 21 after dosing for alanine aminotransferase (ALT) and aspartate aminotransferase (AST) measurements. Treatment with a high-dose urelumab analog (20 mg/kg) resulted in a significant increase in serum ALT levels on days 14 and 21 compared with control groups, while AST levels also exhibited an increasing trend by day 21. These findings indicate a dose-dependent impact of urelumab on liver function in 4-1BB humanized mice. Data are presented as mean ± SEM.

High-dose urelumab analog induced chronic inflammatory response in the liver of 4-1BB humanized mice (B-h4-1BB Mice). 4-1BB humanized mice were divided into three treatment groups and administered hIgG4 isotype control or urelumab analogs at different doses as indicated. On day 21, mice were euthanized and liver tissues were harvested for hematoxylin and eosin (H&E) staining. Representative liver sections from control (G1), low-dose (G2), and high-dose (G3) groups are shown at 100× and 400× magnification. Compared with control and low-dose groups, liver sections from the high-dose urelumab group (G3, 20 mg/kg) displayed prominent inflammatory cell infiltration (arrows), indicating a treatment-associated inflammatory response in the liver. These findings are consistent with the observed changes in serum liver enzyme levels following high-dose urelumab treatment in 4-1BB humanized mice.
Q1: What are 4-1BB humanized mice (B-h4-1BB mice)?
B-h4-1BB mice are a humanized model in which the mouse Tnfrsf9 gene is replaced by the human TNFRSF9 gene. This enables exclusive expression of human 4-1BB (CD137) under endogenous mouse regulatory control.
Q2: Why is humanized 4-1BB (B-h4-1BB) mouse model important for immunotherapy research?
4-1BB receptor is a critical costimulatory immune checkpoint that amplifies T-cell activation, proliferation, and survival. Our humanized 4-1BB (B-h4-1BB) mouse model enables preclinical evaluation of human-specific 4-1BB-targeting therapies, such as agonistic antibodies, by faithfully recapitulating human biology, making it a valuable platform for assessing efficacy and mechanism of action in cancer immunotherapy.
Q3: How is human 4-1BB expression validated?
RT-PCR confirms human TNFRSF9 (4-1BB) mRNA expression exclusively in homozygous B-h4-1BB mice, and flow cytometry verifies exclusive human 4-1BB protein expression on activated immune cells.
Q4: What are the main applications of 4-1BB humanized mice (B-h4-1BB)?
4-1BB humanized (B-h4-1BB) mice are widely used for preclinical evaluation of anti-human 4-1BB immunotherapies, including agonistic antibodies and bispecific antibodies. Key applications include:
Q5: What evidence supports the use of 4-1BB humanized mice (B-h4-1BB) in efficacy studies?
Preclinical studies in 4-1BB humanized (B-h4-1BB) mice demonstrate that anti-human 4-1BB antibodies significantly inhibit MC38 tumor growth. Treatment also remodels the tumor immune microenvironment, marked by increased CD8⁺ T cell infiltration and reduced regulatory T cells, supporting the model's value for antitumor efficacy and immunotherapy mechanism-of-action studies.