
C57BL/6N-Cd38tm3(CD38)Bcgen/Bcgen • 110046
이 페이지에서
CD38 is a 42-kDa glycoprotein, also known as T10. It is an ADP-ribosyl hydrolase expressed on B cells, NK cells, and a subset of T cells, as well as in the brain, muscle, and kidney. In mice, CD38 expression is downregulated on germinal center B cells and plasma cells, whereas this downregulation is not observed in humans. By functioning as both a cyclase and a hydrolase, CD38 mediates lymphocyte activation, adhesion, and the metabolism of cADPR and NAADP. CD31 also serves as a known ligand for CD38.
NAD⁺ is broken down into byproducts that circulate in the bone marrow plasma within the myeloma niche, accumulating various amounts of ADO. Most ADO is taken up by purinergic cell receptors (ADORs) expressed by bone cells or immune cells within the niche. The outcome is either suppression of the anti-tumor activity of immune cells (Teff, NK cells, TAMs) or an increase in regulatory T cells (Tregs), mesenchymal-derived stromal cells (MDSCs), or dendritic cells (DCs), all of which suppress immune activity against tumors.
Increased expression of CD38 is an unfavorable diagnostic marker in chronic lymphocytic leukemia and is associated with enhanced disease progression. CD38 is also the therapeutic target of daratumumab (Darzalex), which is approved for the treatment of multiple myeloma.
Key Advantages
Validation
Application
The CDS of human CD38 encoding the extracellular domain was inserted downstream of a portion of exon 2 of the mouse Cd38 gene in B-hCD38 mice.

Strain specific analysis of CD38 gene expression by RT-PCR. Mouse Cd38 mRNA was detectable only in splenocytes of wild-type (+/+) mice. Human CD38 mRNA was detectable only in homozygous CD38 humanized mice (H/H) but not in wild-type mice.
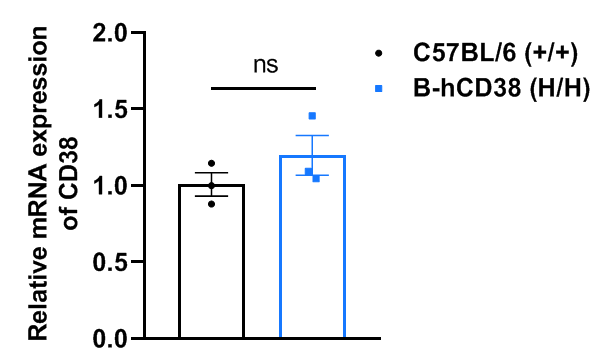
Strain specific analysis of CD38 gene expression in wild-type C57BL/6 and CD38 humanized mice by RT-qPCR. The mRNA expression of CD38 in homozygous CD38 humanized mice (H/H) was similar to that of wild-type C57BL/6 mice (+/+), demonstrating that introduction of human CD38 in place of the mouse counterpart does not alter CD38 expression. Values are expressed as mean ± SEM. Significance was determined by unpaired t-test. *P < 0.05, **P < 0.01, ***P < 0.001.
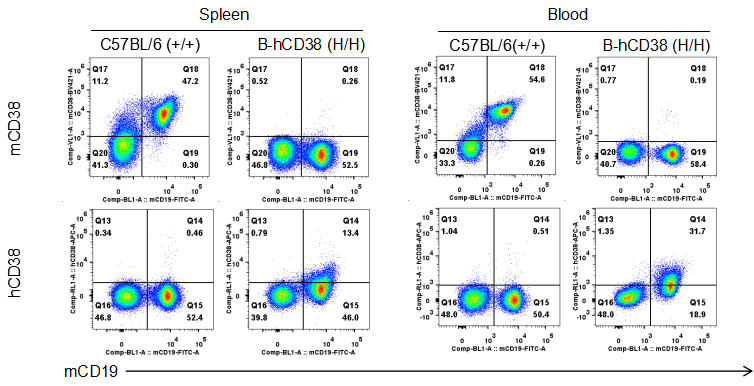
Strain specific CD38 expression in CD38 humanized mice. Splenocytes and blood cells were collected from wild-type and homozygous CD38 humanized mice (H/H) and analyzed by flow cytometry using species-specific anti-CD38 antibodies. Mouse CD38 was detectable in wild-type mice. Human CD38 was exclusively detectable in homozygous CD38 humanized mice, but not in wild-type mice.
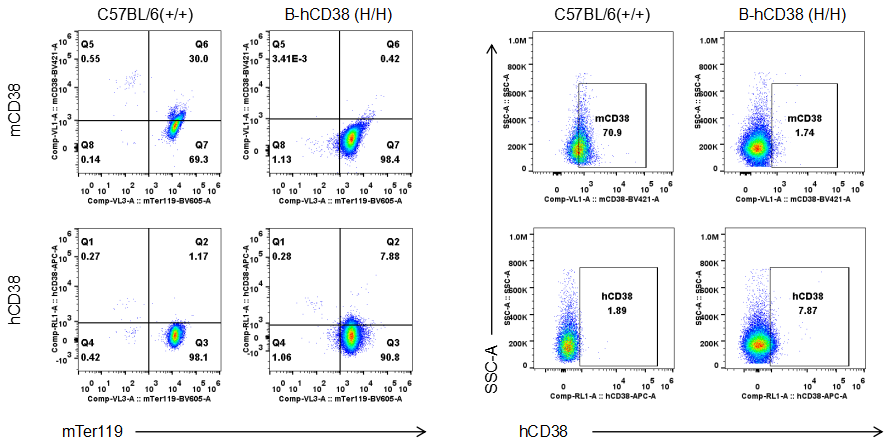
Strain specific CD38 expression in CD38 humanized mice. Blood was collected from wild-type and homozygous CD38 humanized mice (H/H) and analyzed by flow cytometry using species-specific anti-CD38 antibodies. Mouse CD38 was detectable in wild-type mice. Human CD38 was exclusively detectable in homozygous CD38 humanized mice, but not in wild-type mice.
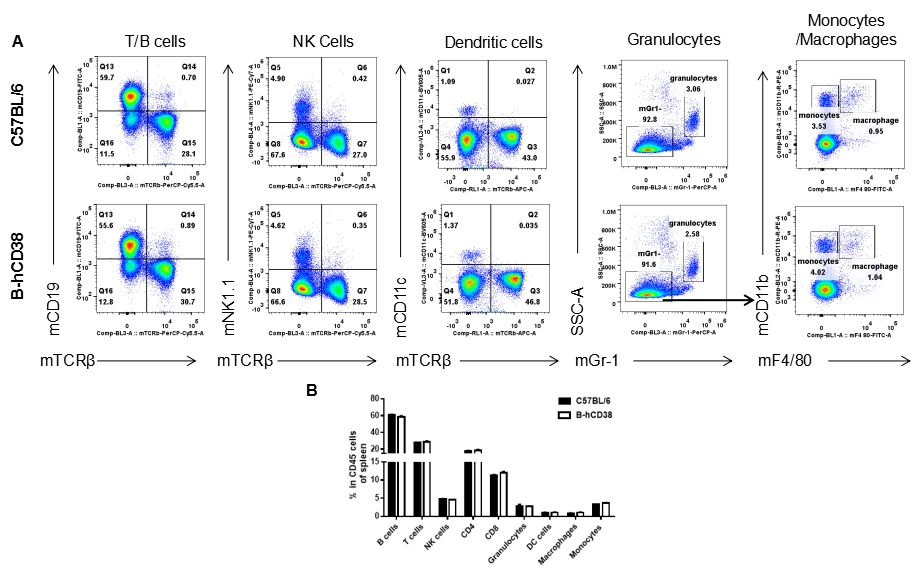
Analysis of splenic leukocyte subpopulations by FACS. Splenocytes were isolated from female C57BL/6 and CD38 humanized mice (n = 3, 6 weeks old) and analyzed by flow cytometry to assess leukocyte subpopulations. (A) Representative FACS plots gated on single live CD45⁺ cells. (B) Results of FACS analysis. Percentages of T cells, B cells, NK cells, monocytes/macrophages, and DCs were similar between homozygous CD38 humanized mice and C57BL/6 mice, demonstrating that introduction of human CD38 does not alter the development, differentiation, or distribution of these cell types in the spleen. Values are expressed as mean ± SEM.
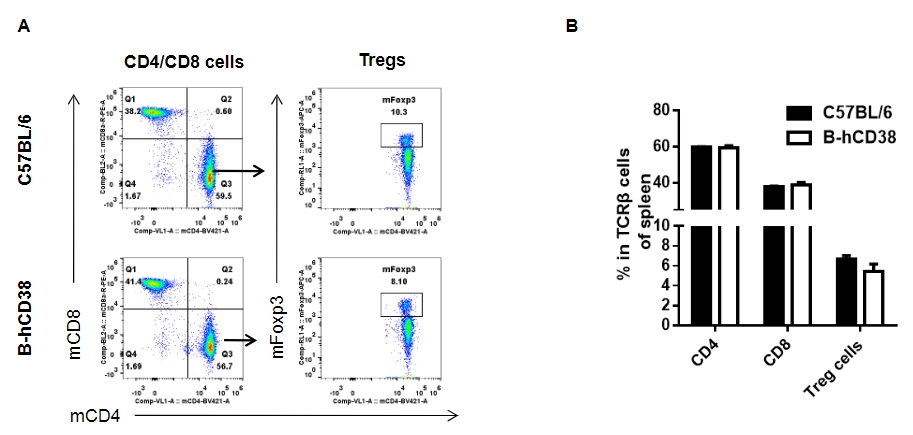
Analysis of splenic T Cell subpopulations by FACS. Splenocytes were isolated from female C57BL/6 and CD38 humanized mice (n = 3, 6 weeks old) and analyzed for T cell subsets. (A) Representative FACS plots gated on TCRβ⁺ T cells. (B) Results of FACS analysis. Percentages of CD8⁺ T cells, CD4⁺ T cells, and Tregs were similar between homozygous CD38 humanized mice and C57BL/6 mice. Values are expressed as mean ± SEM.
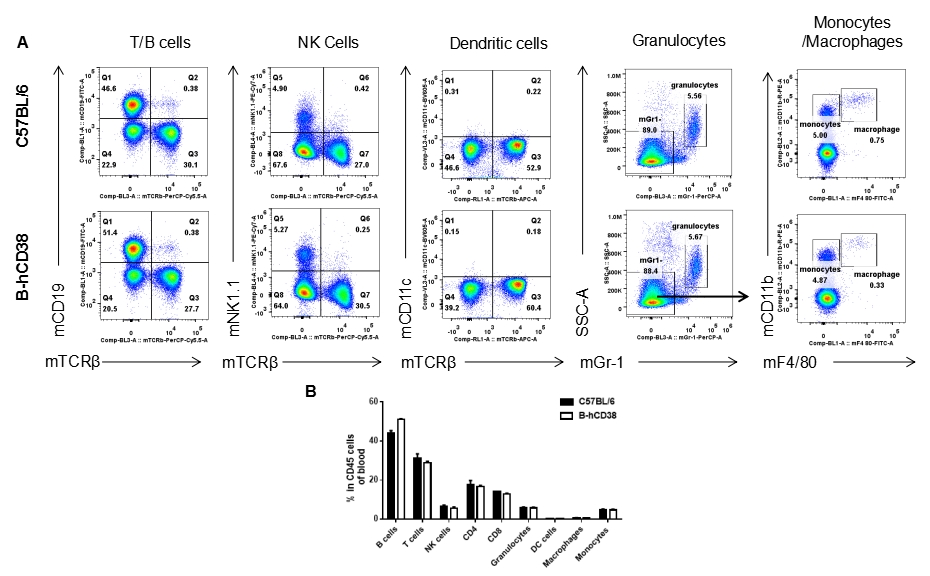
Analysis of blood leukocyte subpopulations by FACS. Blood was isolated from female C57BL/6 and CD38 humanized mice (n = 3, 6 weeks old) and analyzed by flow cytometry to assess leukocyte subpopulations. (A) Representative FACS plots gated on single live CD45⁺ cells. (B) Results of FACS analysis. Percentages of T cells, B cells, NK cells, monocytes/macrophages, and DCs were similar between homozygous CD38 humanized mice and C57BL/6 mice, demonstrating that introduction of human CD38 does not alter the development, differentiation, or distribution of these cell types in the blood. Values are expressed as mean ± SEM.
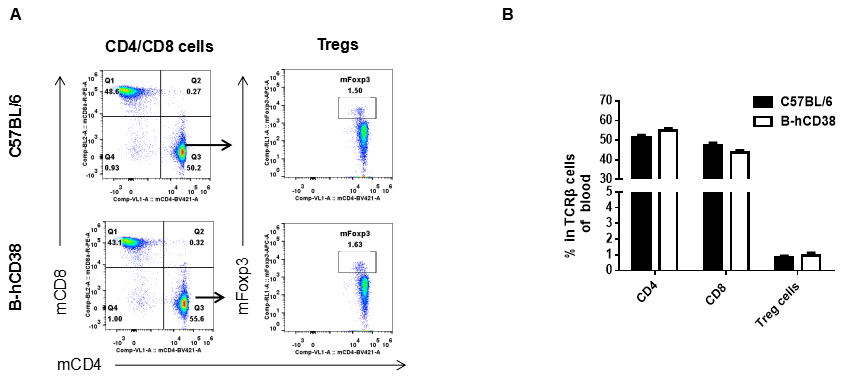
Analysis of blood T cell subpopulations by FACS. Blood was isolated from female C57BL/6 and CD38 humanized mice (n=3, 6 weeks-old) and analyzed by flow cytometry for T cell subsets. (A) Representative FACS plots gated on TCRβ+ T cells and further analyzed. (B) Results of FACS analysis. Percentages of CD8+ T cells, CD4+ T cells and Tregs were similar in homozygous CD38 humanized mice and C57BL/6 mice, demonstrating that introduction of human CD38 in place of its mouse counterpart does not change the overall development, differentiation or distribution of these T cell subtypes in blood. Values are expressed as mean ± SEM.
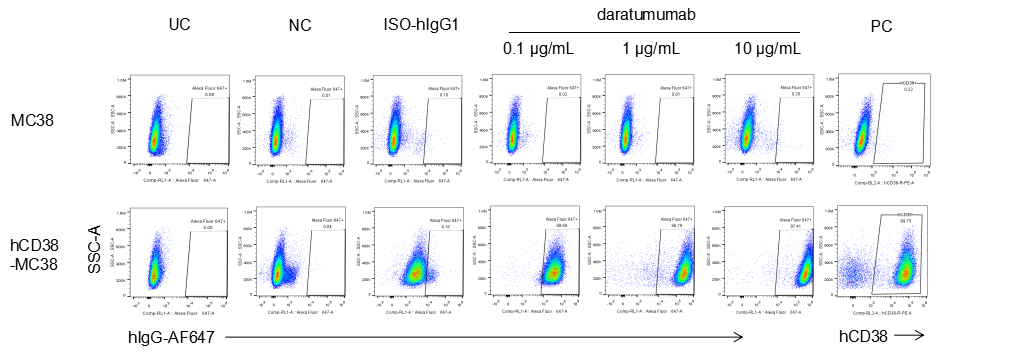
Analysis of B-CAG-hCD38 MC38 by FACS. Flow cytometry analysis of the B-CAG-hCD38 MC38 was performed to assess anti-human CD38 Ab binding. B-CAG-hCD38 MC38 can bind well to anti-hCD38 antibody (daratumumab, in house) vs isotype control.
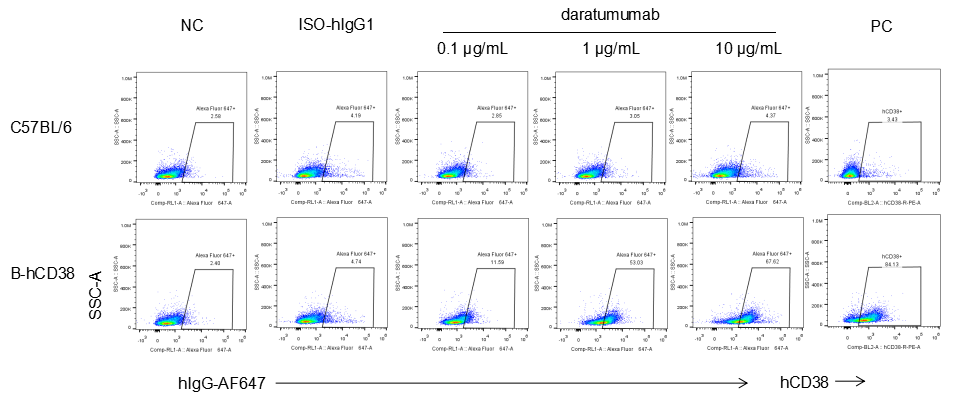
Analysis of splenocytes from CD38 humanized mice by FACS. Splenocytes were isolated from female CD38 humanized mice (6 week-old). Flow cytometry analysis of the splenocytes was performed to assess anti-human CD38 antibody binding with splenocytes. Single live cells were gated for CD19+ population and used for further analysis as indicated. Splenocytes in homozygous CD38 humanized mice can bind well to anti-hCD38 antibody (daratumumab, in house) vs isotype control.
Panel 1: mTER-119, G-anti-hIgG-AF647
Panel 2: Live/Dead, mCD45, mCD3, mCD4, mCD8a, mNK1.1, mFoxp3, G-anti-hIgG-AF647
Panel 3: Live/Dead, mCD45, mCD11b, mCD14, mF4/80, G-anti-hIgG-AF647
Panel 4: Live/Dead, mCD45, mCD3, mCD4, mCD8a, mNK1.1, mFoxp3, hCD38-APC
Panel 5: Live/Dead, mCD45, mCD11b, mCD14, mF4/80, hCD38-APC
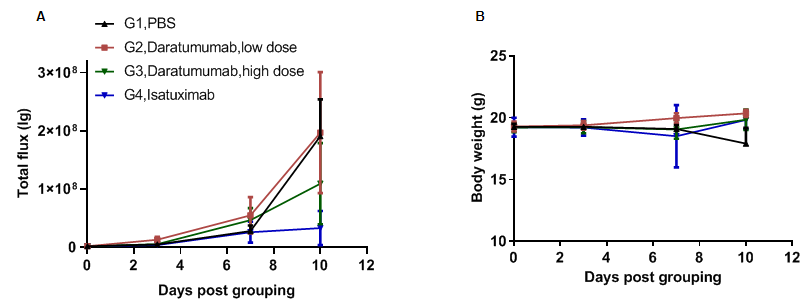
Anti-tumor activity of anti-human CD38 antibody in CD38 humanized mice. (A) Anti-human CD38 antibody (in-house) inhibited B-hCD38-luc E.G7-OVA tumor growth in CD38 humanized mice. Murine T-lymphocytoma B-hCD38-luc E.G7-OVA cells were injected via the tail vein into homozygous CD38 humanized mice (female, 6-week-old, n = 6). Mice were grouped when total flux reached approximately 10⁶ lg, at which point they were treated with the anti-human CD38 antibodies indicated in the panel. (B) Body-weight changes during treatment. As shown in panel A, anti-human CD38 antibodies effectively controlled tumor growth. Values are expressed as mean ± SEM.
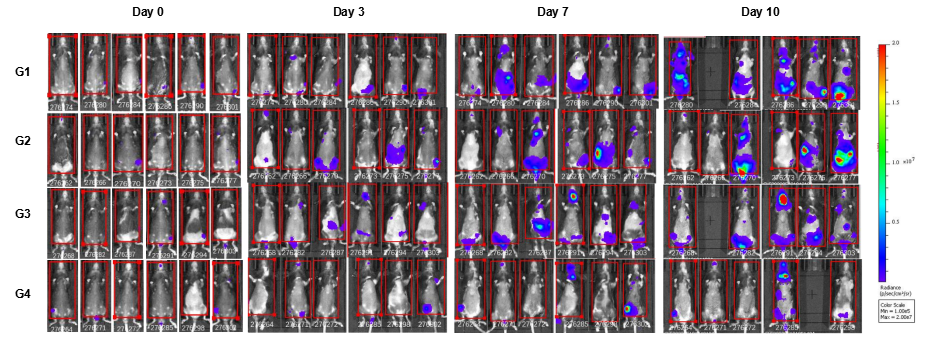
In vivo luciferase images of B-hCD38-Luc E.G7-OVA cells. Mice were grouped when total flux reached approximately 106 Ig, at which time they were treated with anti-human CD38 antibodies. Signal intensity and body weight were measured twice a week. Imaging was performed on days 0, 3, 7 and 10. Values are expressed as mean ± SEM.
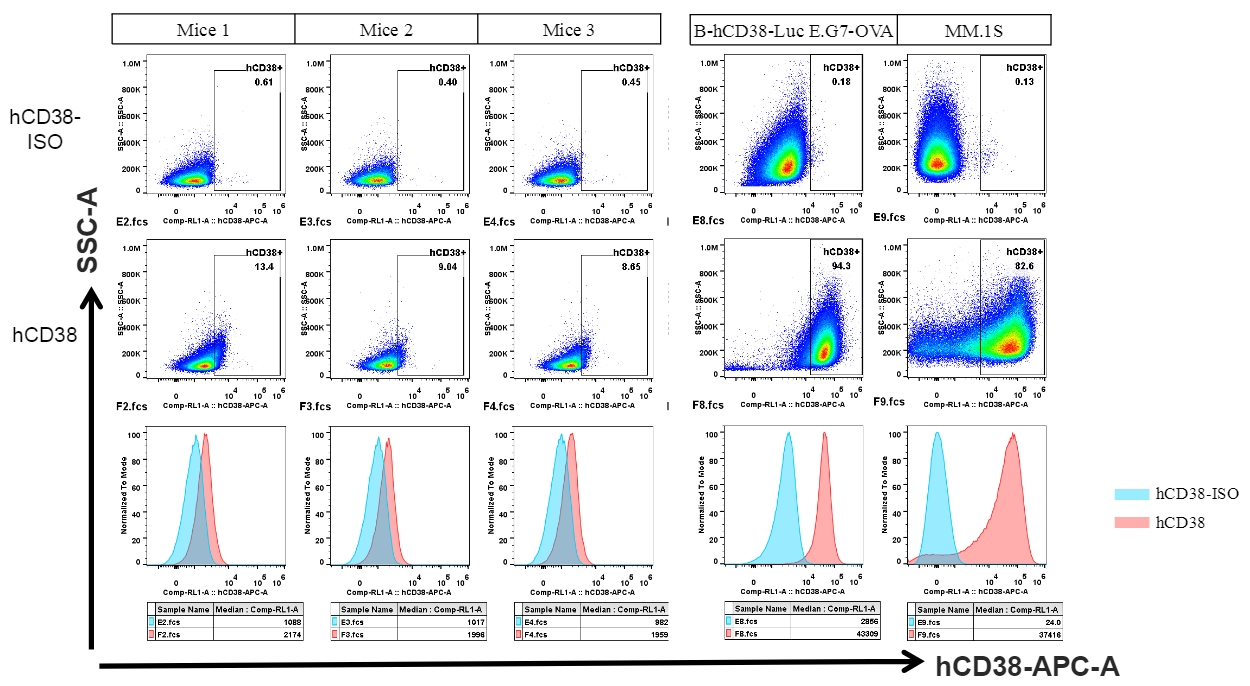
Human CD38 was detected in B cells of spleen from CD38 humanized mice and cell lines.
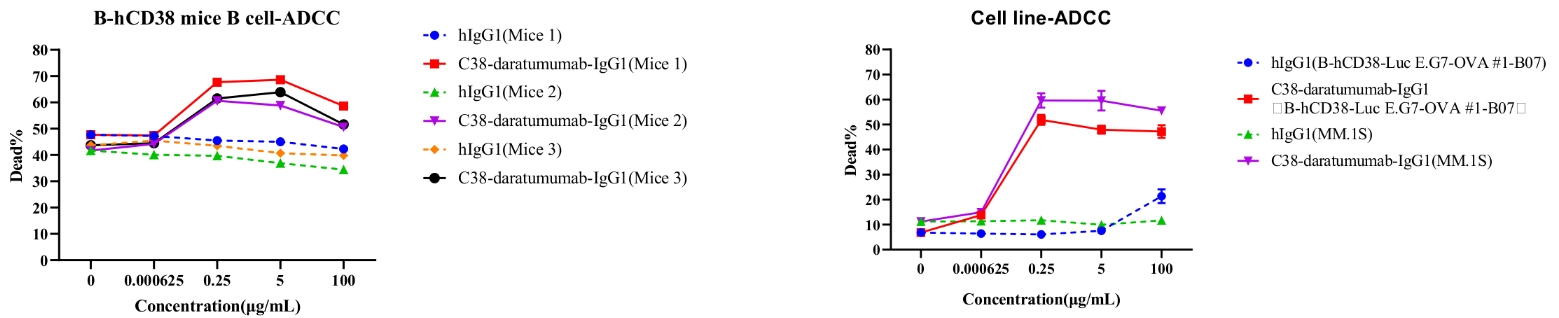
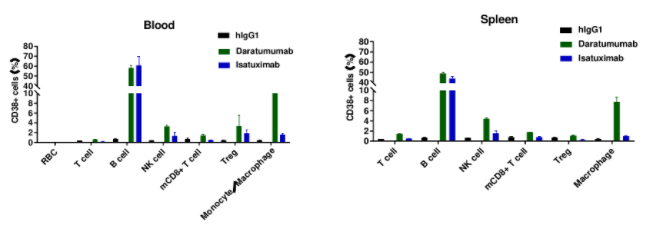
Daratumumab-mediated ADCC against splenic B cells in CD38 humanized mice. ADCC assays were performed against B cells using FcR-TANK cells as effector cells. Antibodies were added at the concentrations shown in the panel. FcR-TANK cells were added to target cells at an E:T ratio of 5:1. After 4 hours at 37 ℃, flow cytometry was performed to assess specific lysis. Results from three different mice are shown (left panel).
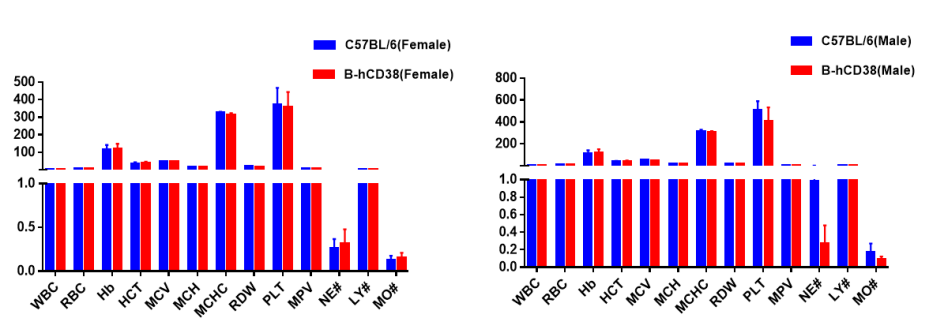
Blood from C57BL/6 and CD38-humanized mice (n = 5; 6-week-old; female and male) was collected and analyzed for complete blood count (CBC). All measurements for CD38 humanized mice were comparable to those of C57BL/6 mice, and no differences were observed between male and female mice, indicating that CD38 humanization does not alter blood cell composition or morphology. Values are expressed as mean ± SEM.
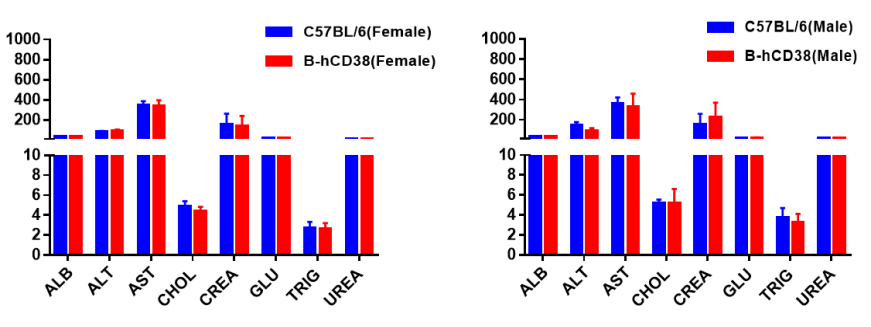
Blood chemistry tests of CD38 humanized mice. Serum from C57BL/6 and CD38 humanized mice (n = 5; 6-week-old; female and male) was collected and analyzed for ALT, AST, and other indicators shown in the panel. There were no differences in any measurements between C57BL/6 and CD38-humanized mice, indicating that CD38 humanization does not alter ALT or AST levels or overall liver health. Values are expressed as mean ± SEM.
Q1: What are CD38 humanized mice used for?
They are designed for testing human CD38-targeting antibodies and studying CD38 biology in vivo under physiologically relevant human expression.
Q2: Do CD38 humanized mice maintain normal immune function?
Yes. Spleen and blood immune subsets are comparable to wild-type mice, indicating preserved immune development.
Q3: Can CD38 humanized mice be used to evaluate daratumumab?
Yes. Splenic B cells from CD38-humanized mice and engineered MC38 cells expressing human CD38 bind daratumumab robustly, enabling efficacy and mechanism-of-action studies.
Q4: Are CD38 humanized mice suitable for ADCC assays?
Absolutely. CD38 humanized mice support robust daratumumab-mediated ADCC against splenic B cells.
Q5: Do CD38 humanized mice have normal baseline health?
Yes. Hematology, blood chemistry, and systemic immune composition have been evaluated and are all within normal ranges.