
C57BL/6-Cd40tm1(CD40)Bcgen/Bcgen • 110009
이 페이지에서
CD40 is a member of the tumor necrosis factor receptor (TNFR) superfamily and is primarily expressed on antigen-presenting cells, including dendritic cells, B cells, macrophages, and monocytes. Interaction between CD40 and its ligand CD40L (CD154) plays a central role in adaptive immunity by promoting antigen presentation, cytokine production, and T-cell priming. Due to its immunostimulatory function, CD40 is a key target for cancer immunotherapy, vaccine adjuvant strategies, and immune modulation research.
In CD40 humanized mice (B-hCD40), the endogenous murine Cd40 gene is replaced with the human CD40 coding sequence to enable species-specific expression of human CD40 under native regulatory control. This humanization design supports translationally relevant evaluation of anti-human CD40 biologics by allowing therapeutic engagement with human CD40 in an in vivo immunocompetent setting while eliminating interference from murine CD40.
Validation data confirm the successful establishment of CD40 humanized mice (B-hCD40), supporting their use as a preclinical model for pharmacodynamic, efficacy, and safety assessment of CD40-targeting therapeutics.
Key Advantages
Validation
Application: CD40 humanized mice (B-hCD40) are used for in vivo evaluation of CD40-targeted antibodies, immune agonists, and combination immunotherapies by enabling human CD40-specific immune activation, antitumor efficacy assessment, and translational safety profiling.
The exons 2-7 of mouse Cd40 gene that encode the extracellular domain were replaced by human CD40 exons 2-7 in CD40 humanized mice (B-hCD40).
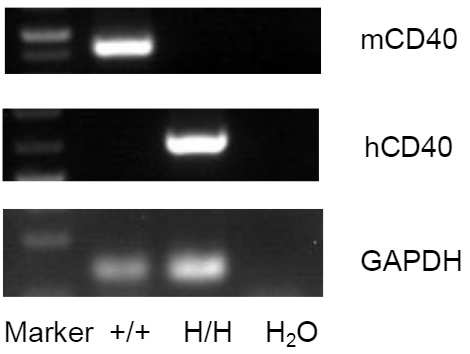
Strain-specific CD40 mRNA expression in CD40 humanized mice (B-hCD40) was evaluated by RT-PCR.
Splenocytes were isolated from wild-type mice (+/+) and homozygous CD40 humanized mice (B-hCD40, H/H), followed by RT-PCR analysis using species-specific primers. Mouse Cd40 mRNA was detected in splenocytes from wild-type mice, whereas human CD40 mRNA was exclusively detected in homozygous CD40 humanized mice (B-hCD40) and was absent in wild-type controls. GAPDH served as an internal control to confirm RNA integrity and assay consistency.
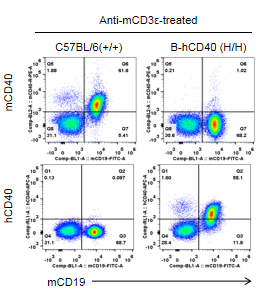
Strain-specific CD40 protein expression in CD40 humanized mice (B-hCD40) was assessed by flow cytometry following in vivo anti-CD3ε stimulation. Splenocytes were isolated from wild-type C57BL/6 mice (+/+) and homozygous CD40 humanized mice (B-hCD40, H/H), treated with anti-CD3ε (7.5 μg/mouse), and analyzed using species-specific anti-CD40 antibodies. Flow cytometry analysis showed that mouse CD40 protein was exclusively detected in wild-type C57BL/6 mice, whereas human CD40 protein was selectively expressed in homozygous CD40 humanized mice (B-hCD40) and was absent in wild-type controls.
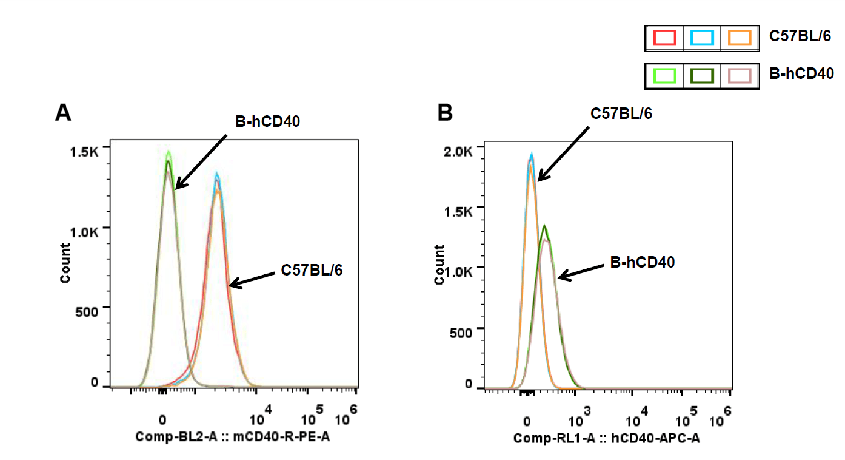
Strain-specific CD40 protein expression in splenic B cells of CD40 humanized mice (B-hCD40) was evaluated by flow cytometry. Splenocytes were isolated from wild-type C57BL/6 mice and homozygous CD40 humanized mice (B-hCD40, H/H) and stained with species-specific CD40 antibodies. Flow cytometric analysis demonstrated that mouse CD40 was readily detectable on B cells from wild-type C57BL/6 mice but was absent in CD40 humanized mice (B-hCD40) (A). In contrast, human CD40 expression was exclusively detected on B cells from homozygous CD40 humanized mice (B-hCD40) and was not observed in wild-type controls (B). These results confirm the successful and specific replacement of endogenous mouse CD40 with human CD40 in the splenic B-cell compartment of CD40 humanized mice (B-hCD40).
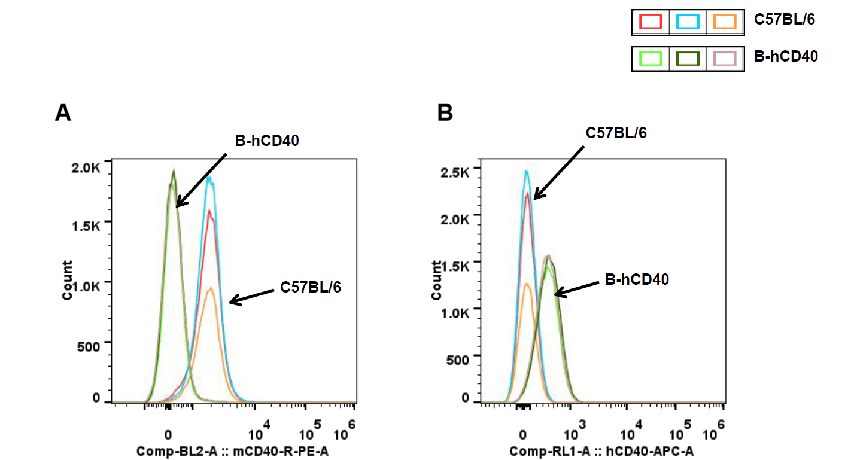
Strain-specific CD40 protein expression in peripheral blood B cells of CD40 humanized mice (B-hCD40) was analyzed by flow cytometry. Peripheral blood samples were collected from wild-type C57BL/6 mice and homozygous CD40 humanized mice (B-hCD40, H/H). B cells were gated and analyzed using species-specific CD40 antibodies. Flow cytometry results show mouse CD40 expression was detectable only in peripheral blood B cells of wild-type C57BL/6 mice) (A), whereas human CD40 expression was exclusively detected in peripheral blood B cells of homozygous CD40 humanized mice (B-hCD40) and demonstrated that was absent in wild-type controls) (B).
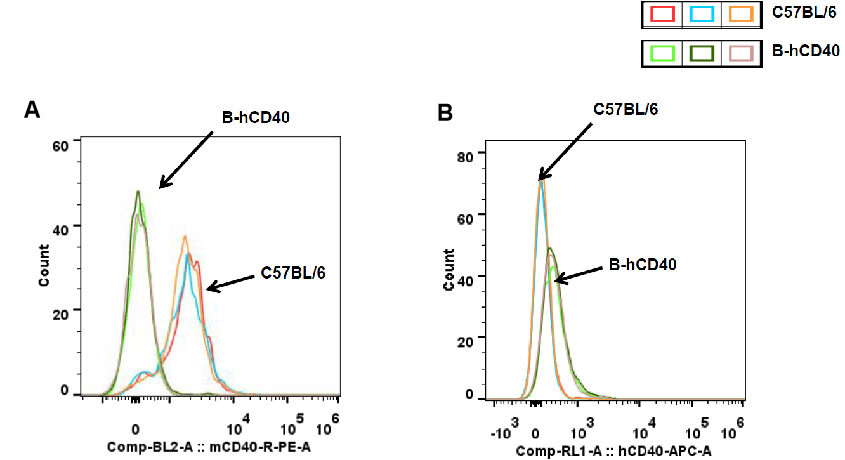
Strain-specific CD40 protein expression in splenic dendritic (DC) cells of CD40 humanized mice (B-hCD40) was analyzed by flow cytometry. Splenocytes were collected from wild-type C57BL/6 mice and homozygous CD40 humanized mice (B-hCD40, H/H), and DC cells were gated and analyzed using species-specific anti-CD40 antibodies. Flow cytometry results demonstrated that mouse CD40 expression was detectable in splenic DC cells of wild-type C57BL/6 mice) (A), whereas human CD40 expression was exclusively detected in splenic DC cells of homozygous CD40 humanized mice (B-hCD40) and was absent in wild-type controls) (B).
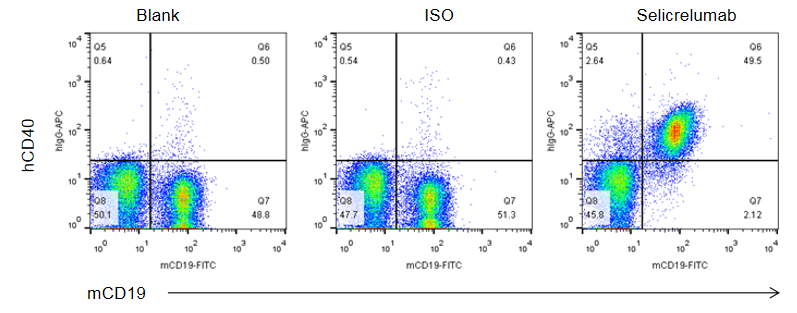
Binding of anti-human CD40 antibodies to B cells was evaluated in CD40 humanized mice (B-hCD40) by flow cytometry. Splenocytes were isolated from female B-hCD40 mice (n = 3) and stained for mCD19 and human CD40 expression. Single live cells were first gated on the CD45⁺ population and subsequently analyzed for CD19⁺ B cells. Flow cytometry analysis demonstrated that human CD40 was clearly detectable on CD19⁺ B cells in B-hCD40 mice, as evidenced by specific binding of the anti-human CD40 antibody selicrelumab (in-house), whereas minimal signal was observed in the blank and isotype control groups.
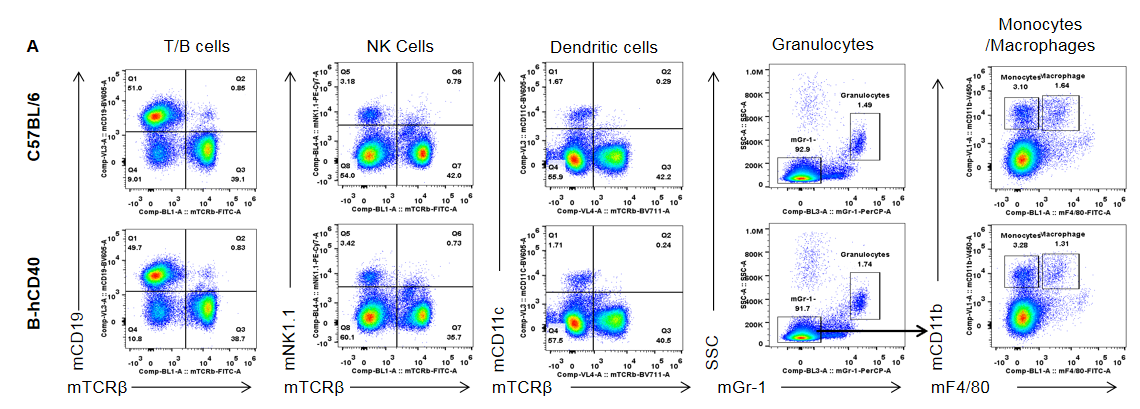
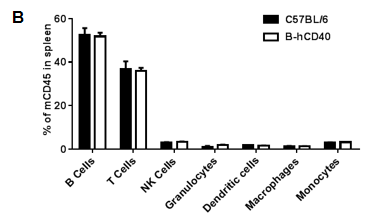
Strain-specific analysis of spleen leukocyte subpopulations in CD40 humanized mice (B-hCD40) by flow cytometry. Splenocytes were isolated from female wild-type C57BL/6 mice and CD40 humanized mice (B-hCD40) (n = 3) and analyzed by flow cytometry to characterize major immune cell subsets. (A) Representative FACS plots show the gating strategy used to identify T cells, B cells, NK cells, dendritic cells, granulocytes, monocytes, and macrophages. Single live CD45⁺ cells were gated and further analyzed using lineage-specific markers as indicated. (B) Quantitative analysis of leukocyte subsets revealed that the proportions of B cells, T cells, NK cells, dendritic cells, granulocytes, monocytes, and macrophages in the spleen of CD40 humanized mice (B-hCD40) were comparable to those observed in wild-type C57BL/6 mice. These results demonstrate that replacement of mouse CD40 with human CD40 does not alter the overall development, differentiation, or distribution of major leukocyte populations in the spleen.
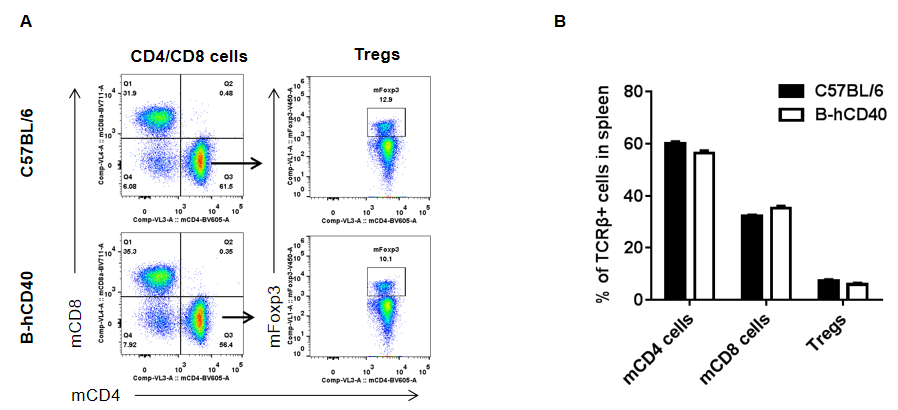
Strain-specific analysis of spleen T cell subpopulations in CD40 humanized mice (B-hCD40) by flow cytometry. Splenocytes were isolated from female wild-type C57BL/6 mice and CD40 humanized mice (B-hCD40) (n = 3) and analyzed by flow cytometry to characterize major T cell subsets in the spleen. (A) Representative FACS plots illustrate the gating strategy used to identify CD3⁺ T cells, followed by discrimination of CD4⁺ T cells, CD8⁺ T cells, and regulatory T cells (Tregs, Foxp3⁺). Single live CD45⁺ cells were gated prior to downstream T cell subset analysis, as indicated. (B) Quantitative analysis of T cell subpopulations shows that the proportions of CD4⁺ T cells, CD8⁺ T cells, and Tregs in the spleen of CD40 humanized mice (B-hCD40) are comparable to those observed in wild-type C57BL/6 mice. These results demonstrate that replacement of mouse CD40 with human CD40 does not alter the overall development, differentiation, or distribution of major T cell subpopulations in the spleen, supporting the immunological integrity of the CD40 humanized mice (B-hCD40) model.
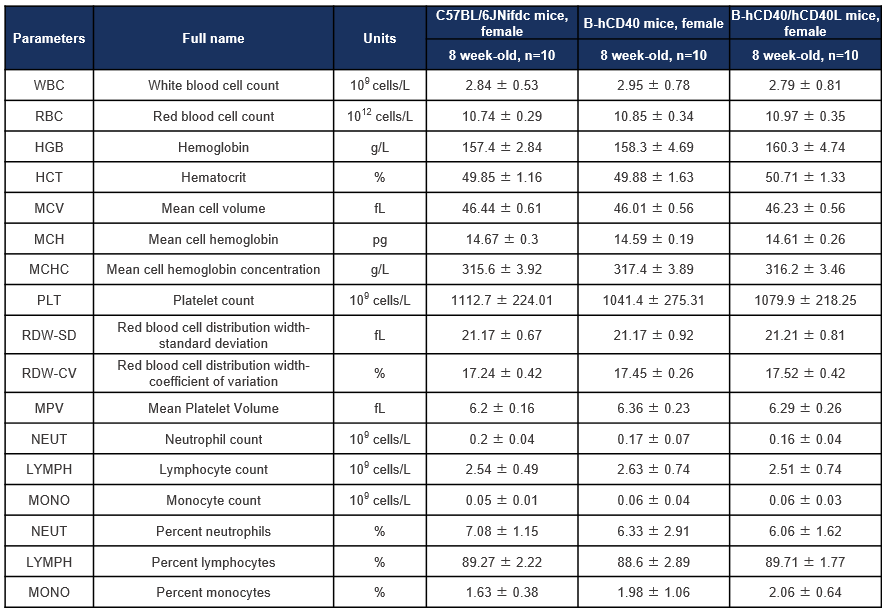
Hematology analysis of CD40 humanized mice (B-hCD40) and CD40/CD40L double-humanized mice (B-hCD40/hCD40L). Complete blood count (CBC) parameters were assessed in female wild-type (C57BL/6JNifdc), CD40 humanized mice (B-hCD40), and CD40/CD40L double-humanized mice (B-hCD40/hCD40L) at 8 weeks of age. Values are expressed as mean ± SD.
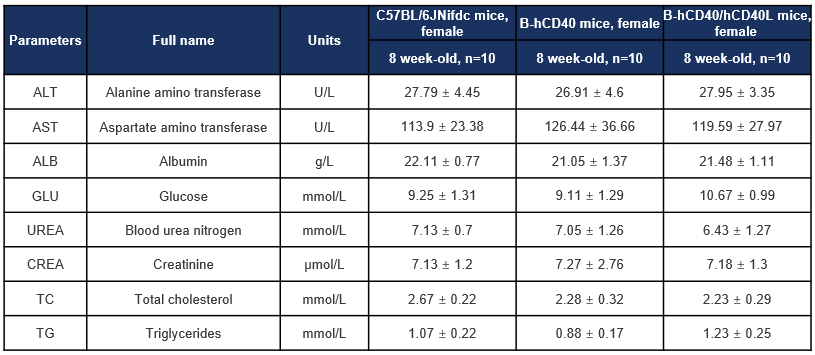
Biochemical analysis was performed to evaluate key serum biochemical parameters in CD40 humanized mice (B-hCD40) and CD40/CD40L double-humanized mice (B-hCD40/hCD40L). Female wild-type C57BL/6JNifdc mice, B-hCD40 mice, and B-hCD40/hCD40L mice (8 weeks old, n = 10 per group) were included in the study. Serum samples were collected and analyzed for liver function markers (ALT, AST, ALB), metabolic indicators (glucose, total cholesterol, triglycerides), and renal function parameters (blood urea nitrogen and creatinine). Values are expressed as mean ± SD.
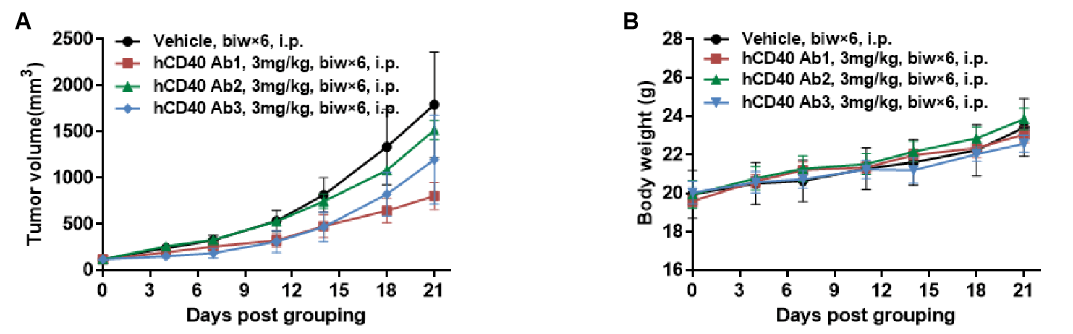
Antitumor activity of anti-human CD40 antibodies in CD40 humanized mice (B-hCD40). Murine colon carcinoma MC38 cells (5 × 10⁵) were subcutaneously implanted into heterozygous CD40 humanized mice (B-hCD40, female, 4 weeks old, n = 5). Mice were randomized when tumor volume reached approximately 100 mm³ and subsequently treated with three anti-human CD40 antibodies (3 mg/kg, biw × 6, i.p.) or vehicle control, as indicated. (A) Tumor growth inhibition. Treatment with anti-human CD40 antibodies significantly suppressed MC38 tumor growth in B-hCD40 mice compared with the vehicle group, demonstrating robust in vivo antitumor efficacy. (B) Body weight monitoring. No significant body weight loss was observed across treatment groups during the study period, indicating acceptable tolerability of anti-human CD40 antibody treatment. Collectively, these results demonstrate that CD40 humanized mice (B-hCD40) provide a robust and translationally relevant in vivo preclinical model for evaluating the antitumor efficacy and safety of anti-human CD40 therapeutic antibodies. Values are expressed as mean ± SEM.
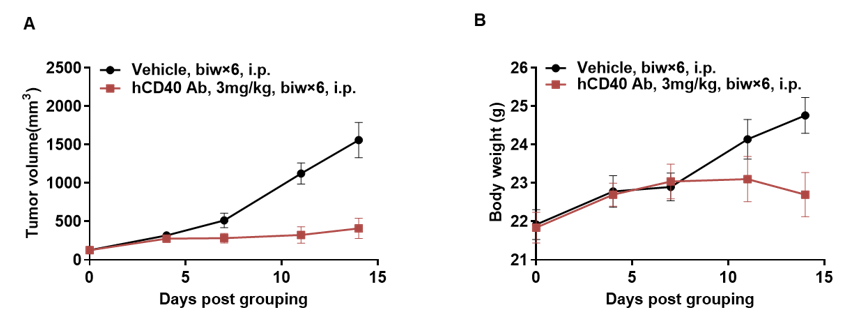
Antitumor activity of anti-human CD40 antibodies in CD40 humanized mice (B-hCD40). Murine colon carcinoma MC38 cells (5 × 10⁵) were subcutaneously implanted into heterozygous CD40 humanized mice (B-hCD40, female, 4 weeks old, n = 5). Mice were randomized when tumor volume reached approximately 100 mm³ and subsequently treated with three anti-human CD40 antibodies (3 mg/kg, biw × 6, i.p.) or vehicle control, as indicated. (A) Tumor growth inhibition. Treatment with anti-human CD40 antibodies significantly suppressed MC38 tumor growth in B-hCD40 mice compared with the vehicle group, demonstrating robust in vivo antitumor efficacy. (B) Body weight monitoring. No significant body weight loss was observed across treatment groups during the study period, indicating acceptable tolerability of anti-human CD40 antibody treatment. Collectively, these results demonstrate that CD40 humanized mice (B-hCD40) provide a robust and translationally relevant in vivo preclinical model for evaluating the antitumor efficacy and safety of anti-human CD40 therapeutic antibodies. Values are expressed as mean ± SEM.
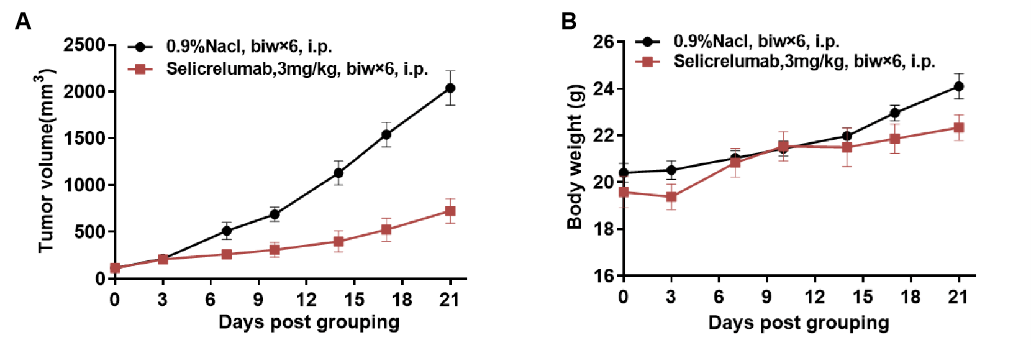
Antitumor activity of anti-human CD40 antibodies in CD40 humanized mice (B-hCD40). Murine colon carcinoma MC38 cells (5 × 10⁵) were subcutaneously implanted into homozygous CD40 humanized mice (B-hCD40; female, 8–9 weeks old, n = 5). When tumor volumes reached approximately 150 ± 50 mm³, mice were randomized and treated with the anti-human CD40 antibody Selicrelumab (in-house, 3 mg/kg, biweekly × 6, i.p.) or vehicle control (0.9% NaCl). (A) Tumor growth inhibition. Treatment with Selicrelumab resulted in a marked suppression of MC38 tumor growth compared with the vehicle group, demonstrating effective antitumor activity mediated through human CD40 signaling in B-hCD40 mice. (B) Body weight monitoring. These results demonstrate that CD40 humanized mice (B-hCD40) provide a robust and translationally relevant preclinical model for in vivo evaluation of anti-human CD40 therapeutic antibodies. Values are expressed as mean ± SEM.
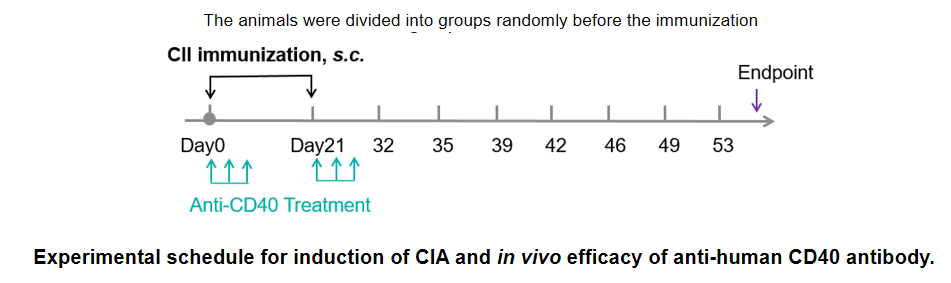
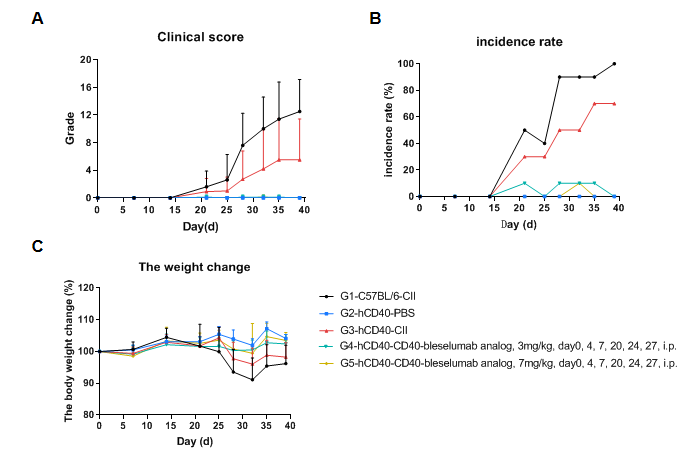
In vivo Efficacy of anti-Human CD40 antibody in a collagen-induced arthritis (CIA) model using CD40 humanized mice (B-hCD40). Mice were randomly assigned to treatment groups prior to immunization. CIA was induced by subcutaneous immunization with type II collagen (CII). Anti-human CD40 antibody treatment was administered according to the indicated schedule, and animals were monitored until the experimental endpoint. (A) Clinical score: Mice in the CIA model groups (G1 & G3) exhibited a progressive increase in clinical arthritis scores, confirming successful induction of the CIA model. Treatment with the anti-human CD40 antibody (G4 & G5) significantly reduced clinical scores compared with the untreated model group, indicating effective disease control. (B) Disease incidence: The incidence of arthritis increased markedly in the model group, whereas anti-human CD40 antibody treatment resulted in a significantly lower maximum incidence rate, demonstrating a protective therapeutic effect. (C) Body weight change: Body weight fluctuations were more pronounced in the CIA model group than in non-model controls. Anti-human CD40 antibody–treated mice showed improved weight stability during the study period. These results demonstrate that anti-human CD40 antibody treatment effectively alleviates disease severity and reduces incidence in the CIA model established in CD40 humanized mice (B-hCD40), supporting the utility of this model for in vivo preclinical evaluation of anti-human CD40 therapeutics.
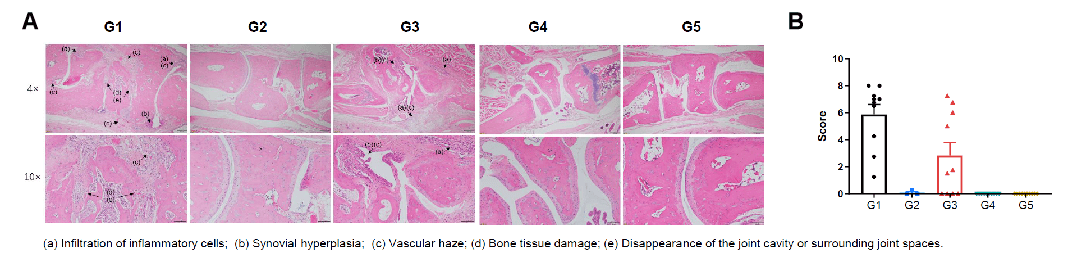
Pathological evaluation of anti-human CD40 antibody bleselumab in a CIA model using CD40 humanized mice (B-hCD40). Histopathological analysis was conducted to evaluate the therapeutic efficacy of the anti-human CD40 antibody bleselumab (in-house) in a collagen-induced arthritis (CIA) model established in CD40 humanized mice (B-hCD40). (A) Representative ankle joint sections from B-hCD40 mice were stained with H&E. (B) Pathological scores were quantitatively assessed. In the non-model control group (G2), CD40 humanized mice (B-hCD40) showed no significant pathological abnormalities, with smooth cartilage surfaces and clearly defined joint cavities. In contrast, mice in the CIA model group (G1 & G3) exhibited typical arthritis-associated pathological features, including infiltration of inflammatory cells, synovial hyperplasia, and vascular haze. Compared with the model group, bleselumab-treated groups (G4 & G5) in CD40 humanized mice (B-hCD40) showed marked attenuation of inflammatory cell infiltration and synovial hyperplasia, accompanied by significant improvement in pathological scores. These findings demonstrate that bleselumab effectively alleviates arthritis-related pathological damage in CD40 humanized mice (B-hCD40), supporting the translational relevance of this model for in vivo evaluation of anti-human CD40 therapies in inflammatory arthritis.
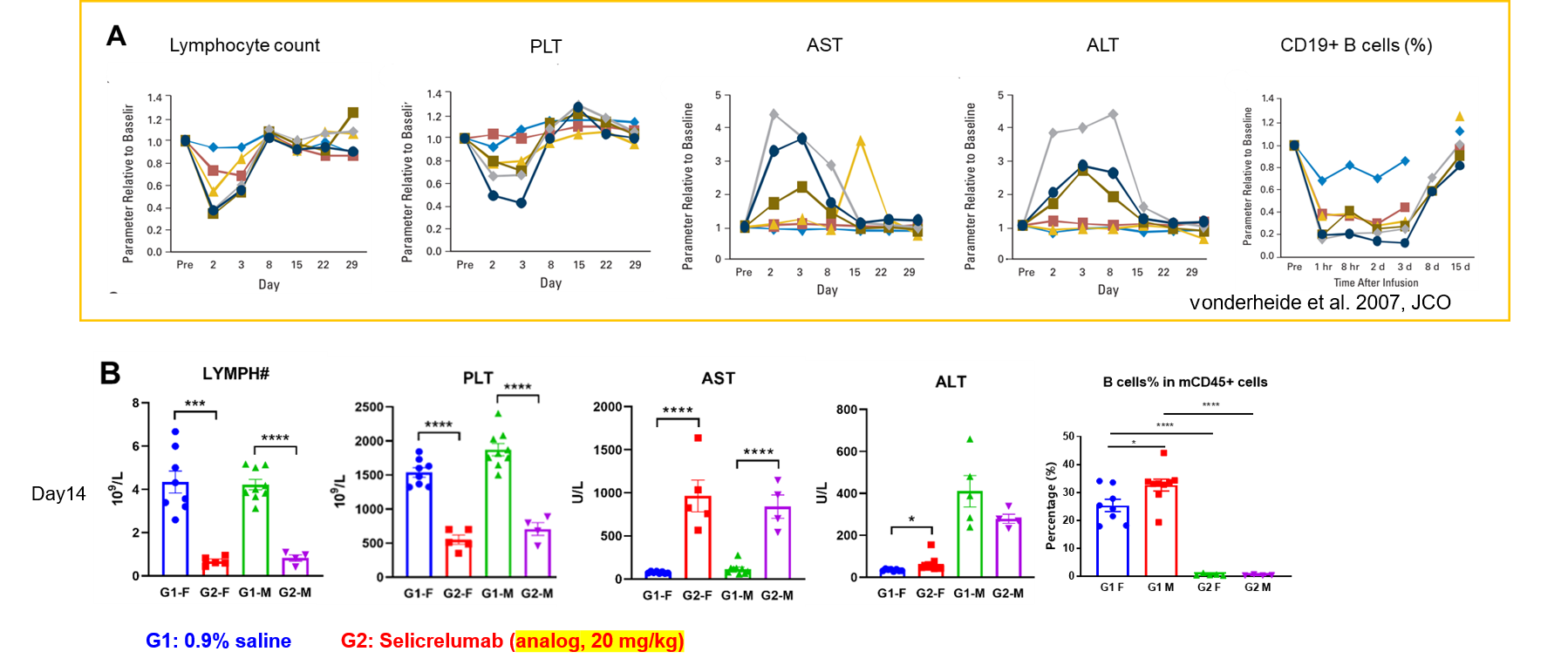
Immune-related adverse event comparison between patients and humanized B-hCD40 mice.(A) After injection of selicrelumab, the clinical patients had obvious hematotoxicity, and the number of peripheral lymphocytes and platelets in the blood decreased. The increase of ALT and AST suggested the occurrence of hepatotoxicity. In addition, CD19+ B cells decreased significantly. (B) B-hCD40 mice of different genders were treated with Selicrelumab, which showed adverse reactions consistent with clinical symptoms.
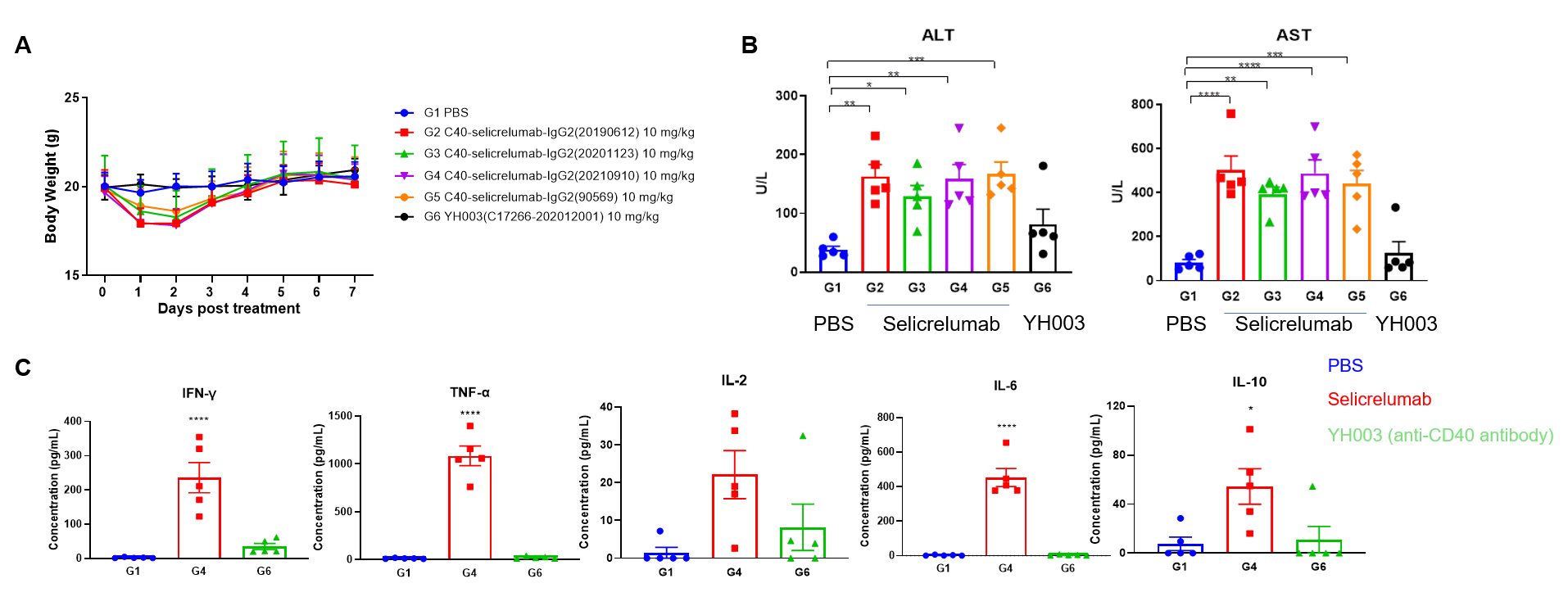
Toxicological profiles of Selicrelumab (analog) in B-hCD40 mice. (A) The body weight of mice in selicrelumab treatment group decreased significantly. (B) Acute phase proteins such as CRP, Amyloid A, Haptoglobin and IL12P40 is increased in response to Selicrelumab in B-hCD40 mice. (C) The mice treated with selicrelumab had obvious cytokine release syndrome, IFN- γ, TNF- α, IL-2, IL-6, IL-10 increased obviously.
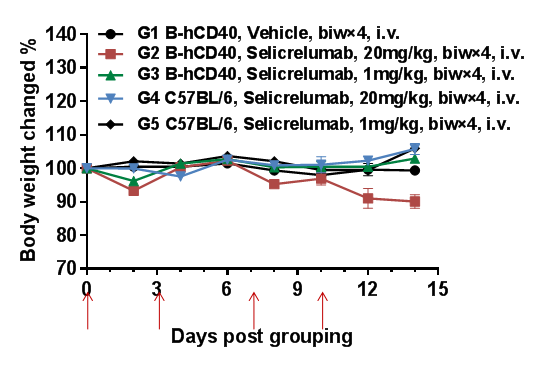
High-dose selicrelumab (anti-human CD40) induces moderate body weight reduction in CD40 humanized mice (B-hCD40), but not in wild-type C57BL/6 mice.
To evaluate the tolerability of anti-human CD40 antibody treatment, homozygous CD40 humanized mice (B-hCD40) and wild-type C57BL/6 mice were treated with vehicle or selicrelumab (in-house) according to the dosing schedule indicated by red arrows (n = 3). Selicrelumab was administered intravenously at doses of 1 mg/kg or 20 mg/kg, twice weekly for four weeks. In CD40 humanized mice (B-hCD40), treatment with selicrelumab at 20 mg/kg resulted in a moderate reduction in body weight compared with vehicle control, whereas the same dosing regimen had no observable effect on body weight in wild-type C57BL/6 mice. Lower-dose selicrelumab (1 mg/kg) did not cause significant body weight changes in either genotype. Values are expressed as mean ± SEM.
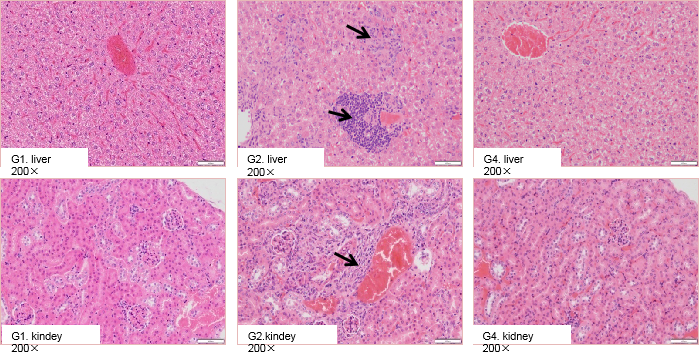
High-dose selicrelumab treatment induced pathological changes in the liver and kidney of CD40 humanized mice (B-hCD40). Homozygous B-hCD40 mice and wild-type C57BL/6 mice were treated with PBS or selicrelumab (n = 3), and tissue pathology analysis was performed on day 14 after treatment. In CD40 humanized mice (B-hCD40) mice, selicrelumab administered at 20 mg/kg resulted in increased lymphocyte infiltration in both liver and kidney tissues, as indicated by arrows. In contrast, the same treatment did not induce detectable pathological changes in the liver or kidney of wild-type C57BL/6 mice.
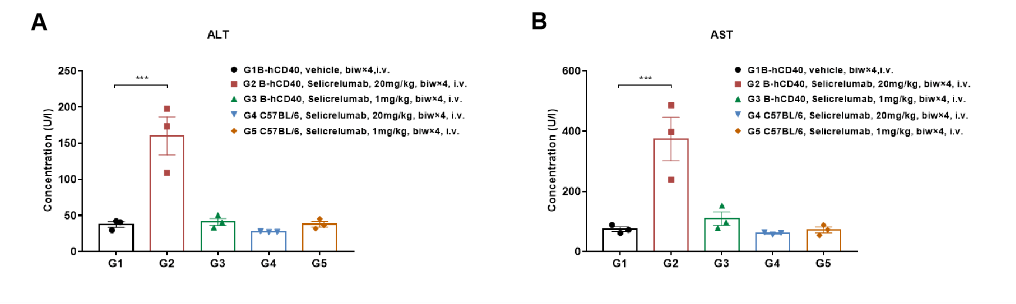
High-dose selicrelumab treatment resulted in significant changes in serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST) levels in CD40 humanized mice (B-hCD40). Homozygous B-hCD40 mice and wild-type C57BL/6 mice were treated with PBS or selicrelumab (in-house) according to the indicated dosing schedule (n = 3). Blood biochemical analysis was performed 24 hours after termination of the efficacy evaluation experiment. (A) Serum alanine aminotransferase (ALT) levels.(B) Serum aspartate aminotransferase (AST) levels.
In B-hCD40 mice, selicrelumab administered at 20 mg/kg caused a significant increase in ALT and AST levels (A, B) compared with PBS-treated controls, but not in wild-type C57BL/6 mice. These findings indicate a target-dependent liver toxicity profile associated with high-dose anti-human CD40 antibody treatment. Values are expressed as mean ± SEM.
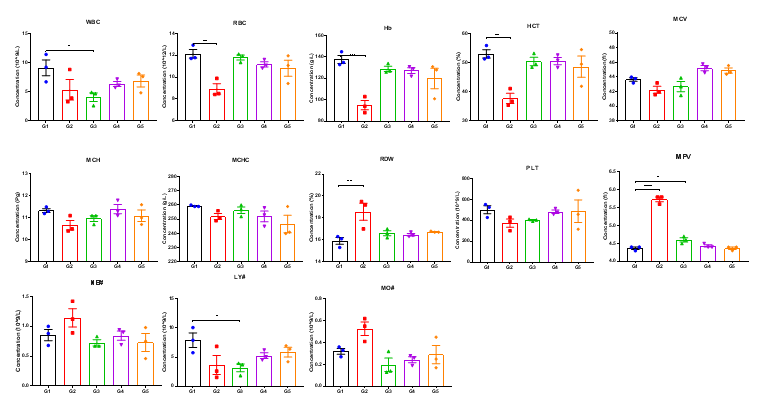
High-dose human CD40 antibody treatment induced significant alterations in hematological parameters in CD40 humanized mice (B-hCD40). Homozygous CD40 humanized mice (B-hCD40) and wild-type C57BL/6 mice were treated with PBS or selicrelumab (in-house) as indicated in the graph (n = 3). Complete blood count (CBC) analysis was performed 24 hours after the last dose on day 11. In CD40 humanized mice (B-hCD40) , selicrelumab administered at 20 mg/kg resulted in a significant reduction in red blood cell count (RBC) and hemoglobin (Hb) levels, accompanied by decreases in hematocrit (HCT) and lymphocyte percentage (LY#), as well as increases in red cell distribution width (RDW) and mean platelet volume (MPV), compared with PBS-treated controls. In contrast, the same treatment regimen did not induce significant changes in CBC parameters in wild-type C57BL/6 mice. Values are expressed as mean ± SEM.
Q1: What is the CD40 humanized mouse model (B-hCD40 mice)?
The CD40 humanized mouse model (B-hCD40 mice) is a genetically engineered mouse in which the endogenous murine Cd40 gene is replaced by the human CD40 coding sequence, enabling physiological expression of human CD40 under native regulatory control. This model allows in vivo evaluation of human CD40–targeting therapeutics within an intact immune system.
Q2: Why is CD40 an important immunotherapy target?
CD40 is a costimulatory receptor expressed primarily on antigen-presenting cells, including dendritic cells, B cells, and macrophages. CD40 signaling plays a central role in antigen presentation, T-cell priming, and adaptive immune activation, making it a key target for cancer immunotherapy, vaccine development, and immune modulation studies.
Q3: What advantages do CD40 humanized mice (B-hCD40 mice) offer over wild-type mice?
Unlike wild-type mice, B-hCD40 mice express human CD40 instead of murine CD40, enabling direct assessment of human CD40 agonistic or antagonistic antibodies in vivo. This avoids species cross-reactivity limitations and provides more translationally relevant pharmacodynamic and safety data.
Q4: Can CD40 humanized mouse model (B-hCD40 mice) be used to evaluate anti-human CD40 antibodies?
Yes. B-hCD40 mice are specifically designed for in vivo efficacy and safety evaluation of anti-human CD40 antibodies, including agonists and immune modulators. The model supports assessment of immune activation, antitumor efficacy, cytokine responses, and potential immune-related toxicities.
Q5: What applications are CD40 humanized mouse models (B-hCD40 mice) suitable for?
B-hCD40 mice are suitable for preclinical studies including cancer immunotherapy evaluation, immune agonist development, antibody pharmacodynamics, combination therapy studies, and immune safety assessment. They are also applicable to mechanistic studies of CD40-mediated immune activation.