
BALB/cCrSlcNifdc-Il33tm1(IL33)Bcgen Tslptm1(TSLP)Bcgen Crlf2tm2(CRLF2)Bcgen/Bcgen • 113713

| Product name | B-hIL33/hTSLP/hTSLPR mice(C) |
|---|---|
| Catalog number | 113713 |
| Strain name | BALB/cCrSlcNifdc-Il33tm1(IL33)Bcgen Tslptm1(TSLP)Bcgen Crlf2tm2(CRLF2)Bcgen/Bcgen |
| Strain background | BALB/cCrSlcNifdc |
| NCBI gene ID | 90865,85480,64109 (Human) |
| Aliases | DVS27; IL1F11; NF-HEV; NFEHEV; C9orf26; CRL2; TSLPR; CRLF2Y |
Gene targeting strategy for B-hIL33/hTSLP/hTSLPR mice(C). The exons 2-8 of mouse Il33 gene that encode the full-length protein are replaced by human IL33 exons 2-8 in B-hIL33/hTSLP/hTSLPR mice(C). The exons 1-5 of mouse Tslp gene that encode the whole molecule (ATG to STOP codon) are replaced by human counterparts in B-hIL33/hTSLP/hTSLPR mice(C). The promoter, 5’UTR and 3’UTR region of the mouse gene are retained. The human TSLP expression is driven by endogenous mouse Tslp promoter, while mouse Tslp gene transcription and translation will be disrupted; A chimeric CDS that encodes human TSLPR extracellular domain, transmembrane and mouse Tslpr cytoplasmic domain, followed by mouse 3’UTR-STOP is inserted right after mouse Tslpr signal peptide sequence to replace part of the exon 2 of mouse Tslpr gene. The chimeric TSLPR protein expression will be driven by endogenous mouse Tslpr promoter, while mouse Tslpr gene transcription and translation will be disrupted.
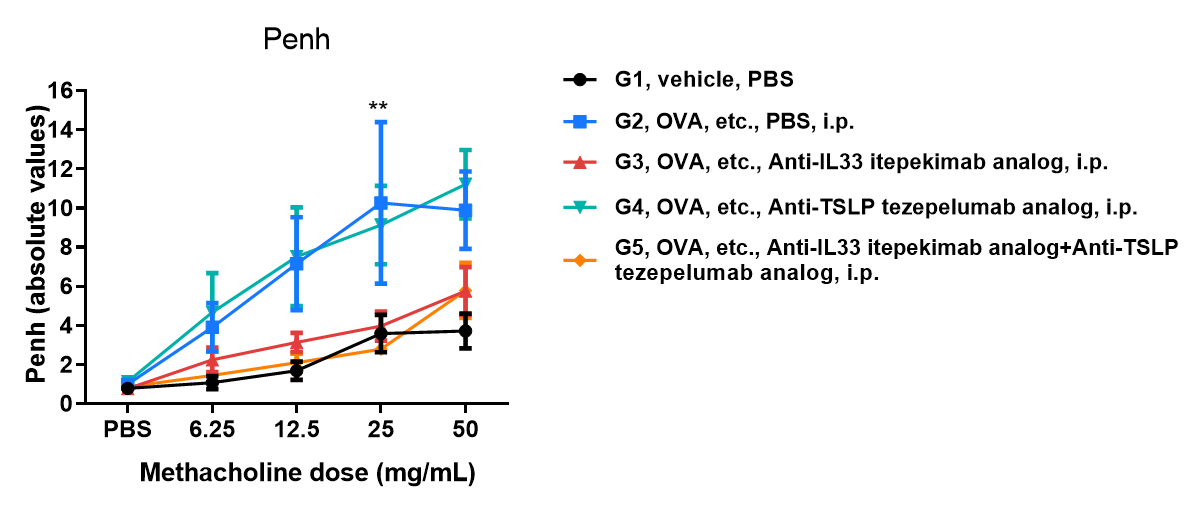
Measurement of enhanced pause (Penh) by whole-body plethysmography. Airway responses following the exposure to increasing doses of methacholine (MCh) were measured for each mouse 24 hr after the final allergen or PBS exposure using the whole-body plethysmography. The y-axis represents the Penh absolute value. Increasing doses of methacholine were administered by aerosols. B-hIL33/hTSLP/hTSLPR mice(C) (female, 7-9-week-old, n=5) exposed to OVA showed a significant increase in airway hyperreactivity to MCh when compared to exposed PBS inhalation (n=5) (*P<0.05, *** P<0.01). Penh values were significantly decreased treated with either anti-IL33 antibody (Itepekimab analog, synthesized in-house) or a combination therapy (anti-IL33 antibody plus anti-TSLP antibody), compared to the PBS-treated group after allergen exposure (n=5) after allergen exposure [MCh doses of 6.25, 12.5, 25 and 50mg/mL (***P<0.001)]. Values are expressed as mean ± SEM. Significance is determined by two-way ANOVA test. *P < 0.05, **P < 0.01, ***P < 0.001.
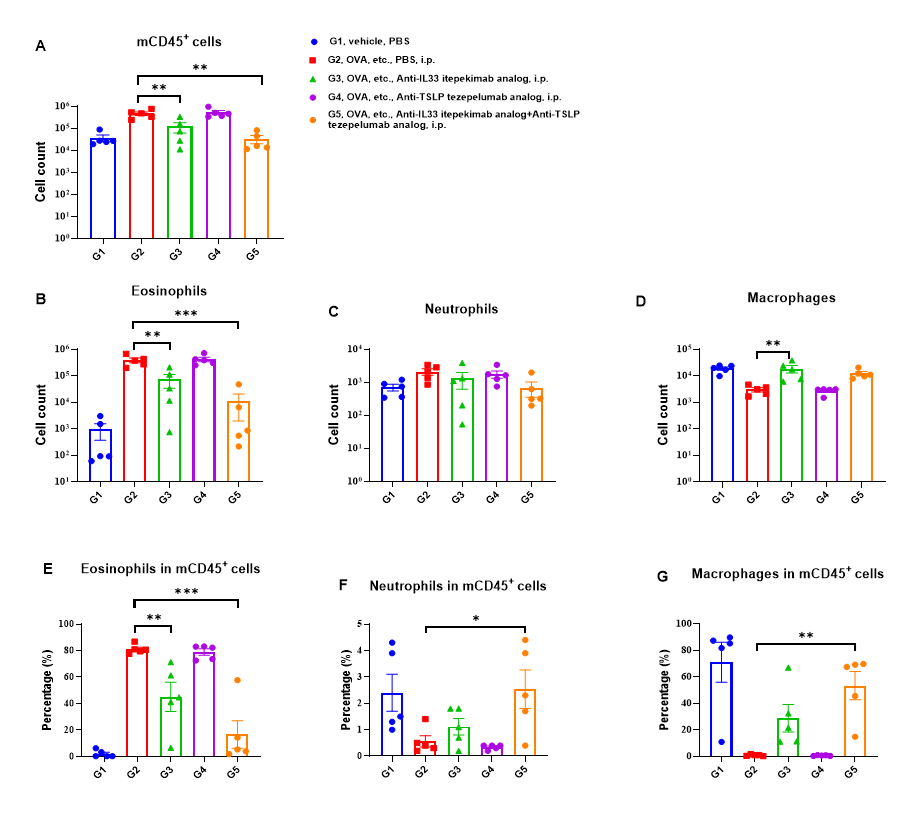
Analysis of immune cells in BALF. B-hIL33/hTSLP/hTSLPR mice(C) (female, 7-9-week-old, n=5) were immunized with OVA etc. to induce asthma. Anti-human IL33 antibody (Itepekimab analog, synthesized in-house) and anti-human TSLP antiboday (Tezepelumab analog, synthesized in-house) were intraperitoneally injected to B-hIL33/hTSLP/hTSLPR mice(C). (A&B) The number of CD45+ cells and eosinophils of BALF in the Itepekimab and the combination therapy group of Itepekimab and Tezepelumab treated groups decreased significantly compared with the OVA etc.-induced PBS treated group. Values are expressed as mean ± SEM. Significance is determined by two-way ANOVA test. *P < 0.05, **P < 0.01, ***P < 0.001.
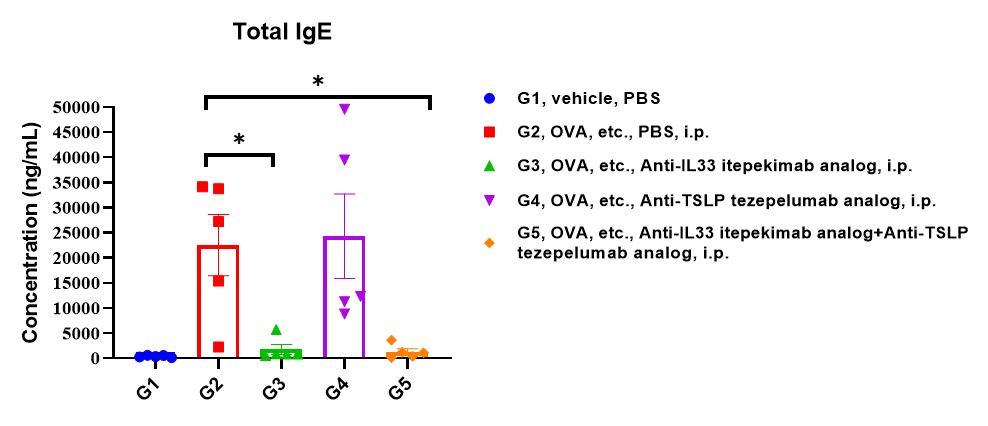
Analysis of mouse total IgE in serum. B-hIL33/hTSLP/hTSLPR mice(C) (female, 7-9-week-old, n=5) were immune zed with OVA etc.to induce asthma. Anti-human IL33 antibody (Itepekimab analog, synthesized in-house) and anti-human TSLP antiboday (Tezepelumab analog, synthesized in-house) were intraperitoneally injected to B-hIL33/hTSLP/hTSLPR mice(C). Serum was collected at the study endpoint. IgE level was analyzed by ELISA. The results showed that the levels of total IgE in mice treated with Itepekimab, and the combination therapy group of Itepekimab and Tezepelumab treated groups showed a significant reduction compared with untreated mice. Values are expressed as mean ± SEM. Significance is determined by two-way ANOVA test. *P < 0.05, **P < 0.01, ***P < 0.001.
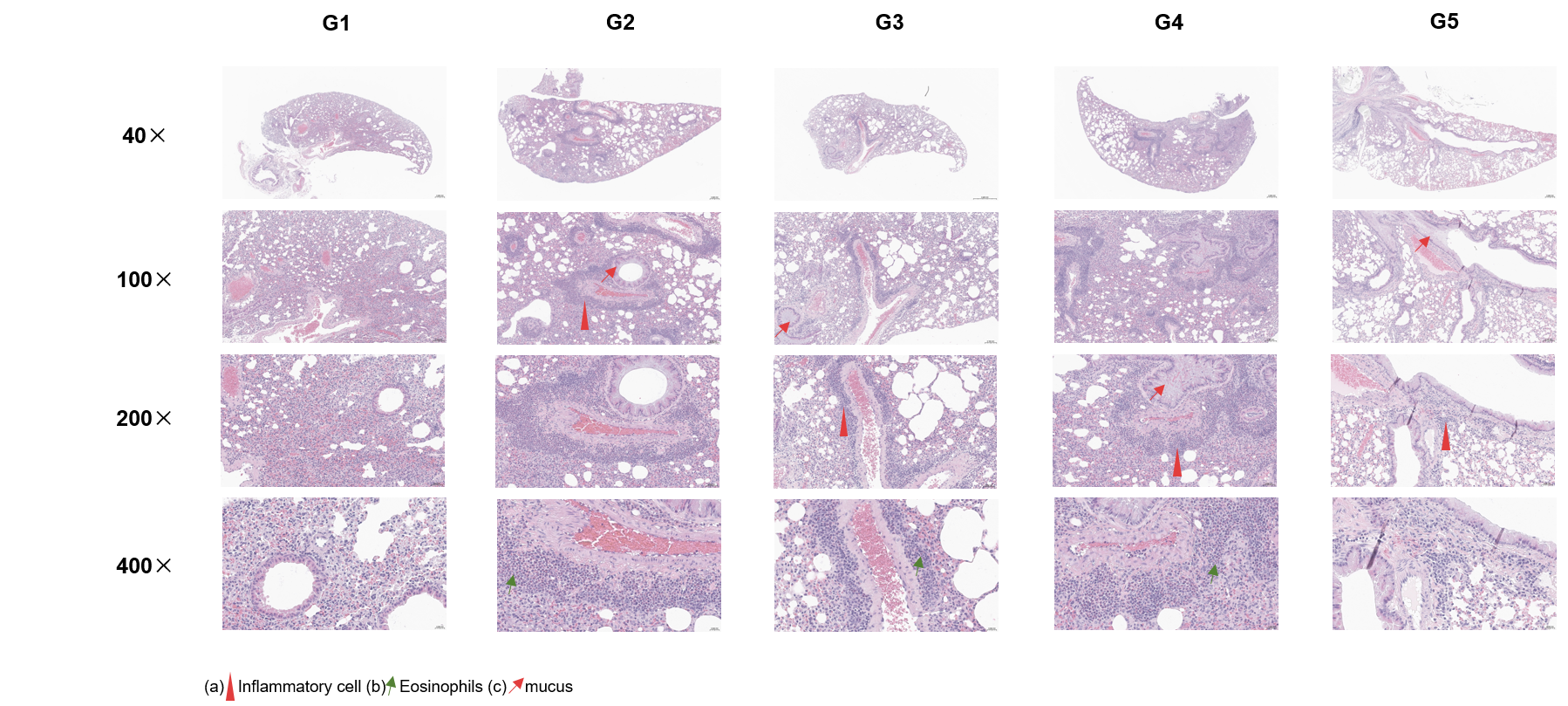
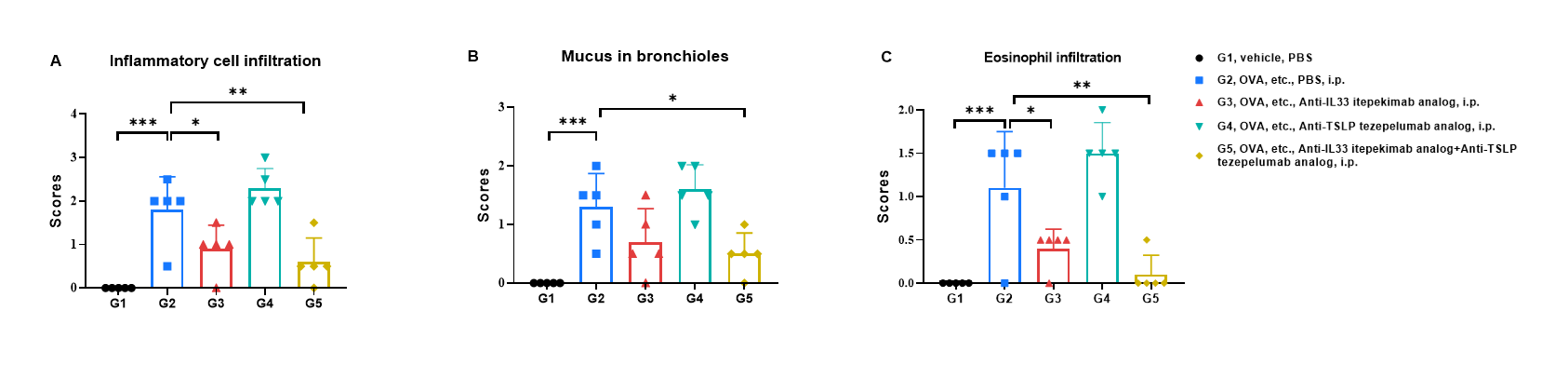
H&E staining of asthma-like model in B-hIL33/hTSLP/hTSLPR mice(C). Lung tissues were collected at the study endpoint and analyzed with H&E staining. The results showed that the groups of mice treated with Itepekimab and the combination therapy group of Itepekimab and Tezepelumab in inflammatory infiltration and mucus secretion in lung tissue were lower than that in untreated mice, indicating that B-hIL33/hTSLP/hTSLPR mice(C) provide a powerful preclinical model for in vivo evaluation of anti-human IL33 antibodies and anti-human TSLP antibodies. Values are expressed as mean ± SEM. Significance is determined by unpaired t-test. *P < 0.05, **P < 0.01, ***P < 0.001.