
C57BL/6JNifdc-Pahtm1(PAH*R243Q)Bcgen/Bcgen • 113494
Gene targeting strategy for B-hPAH*R243Q mice. The exon 7 of mouse Pah gene was replaced by human PAH exon 7 with the R243Q mutation in B-hPAH*R243Q mice.
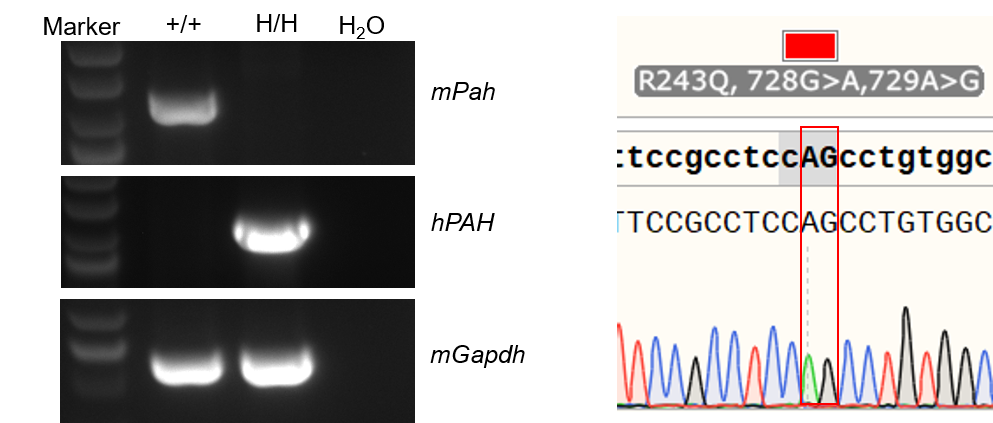
Strain specific analysis of PAH mRNA expression in wild-type C57BL/6JNifdc mice and B-hPAH*R243Q mice by RT-PCR. Liver RNA were isolated from wild-type C57BL/6JNifdc mice (+/+) and homozygous B-hPAH*R243Q mice (H/H), then cDNA libraries were synthesized by reverse transcription, followed by PCR with mouse or human PAH primers. Mouse Pah mRNA was only detectable in wild-type mice. Human PAH mRNA was exclusively detectable in homozygous B-hPAH*R243Q mice. Point mutations in homozygous B-hPAH*R243Q mice were confirmed via Sanger Sequencing.
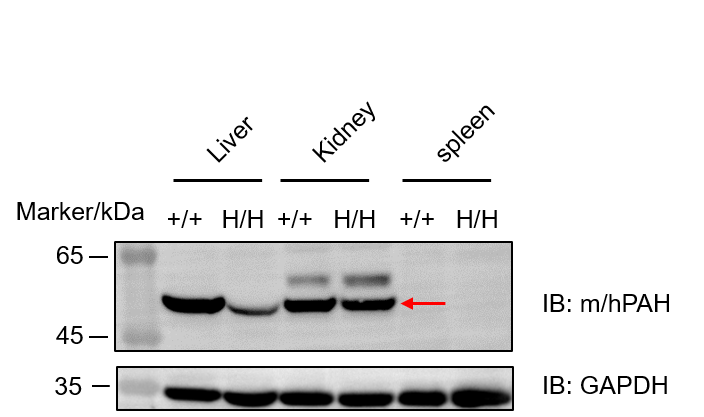
Western blot analysis of PAH protein expression in homozygous B-hPAH*R243Q mice. Various tissue lysates were collected from wild-type C57BL/6JNifdc mice (+/+) and homozygous B-hPAH*R243Q mice (H/H), and then analyzed by western blot with anti-PAH antibody (abcam, ab178430). 40 μg total proteins were loaded for western blot analysis. PAH protein expression was detected in the liver and kidney of both wild-type mice and homozygous B-hPAH*R243Q mice (indicated by the red arrow), as the antibody used showed cross-reactivity between human and mouse PAH. Moreover, PAH protein expression in liver from wild-type mice was higher than that from homozygous B-hPAH*R243Q mice.
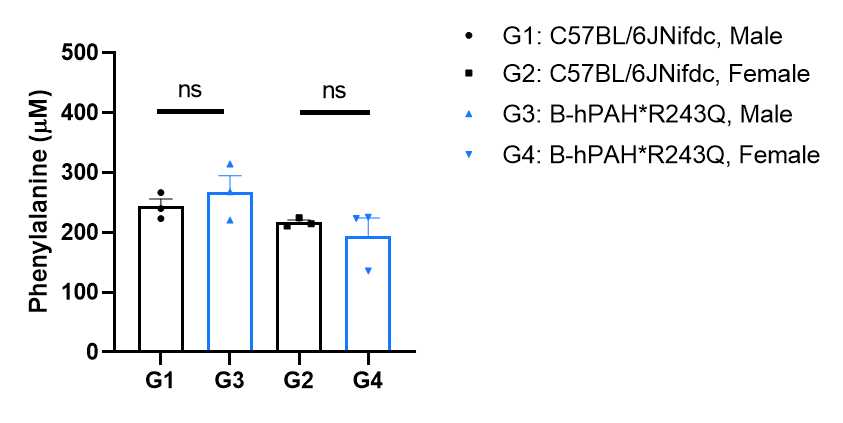
Phenylalanine concentration analysis in homozygous B-hPAH*R408W mice. Serum sample were collected from wild-type C57BL/6JNifdc mice (+/+, n=3 per sex, 8-week-old) and homozygous B-hPAH*R243Q mice (H/H, n=3 per sex, 8-week-old). All of the mice were maintained on standard mouse chow. Serum phenylalanine (Phe) concentration was measured using phenylalanine assay kit (abcam, ab241000). The serum Phe concentration in homozygous B-hPAH*R243Q mice (~230 μM) was similar with those in wild-type mice. Values are expressed as mean ± SEM. Significance was determined by unpaired t test. *P < 0.05, **P < 0.01, ***P < 0.001.