
C57BL/6-Cd8atm1(CD8A)BcgenCd8btm1(CD8B)Bcgen/Bcgen • 112811
이 페이지에서
Key Advantages
Validation
Application
In CD8 humanized mice, exons 1–3 and part of exon 4 of the mouse Cd8a gene—encoding the signal peptide and extracellular domain—are replaced with human sequences. The mouse genomic regions encoding the transmembrane and cytoplasmic domains, along with the promoter, 5'UTR, and 3'UTR, are retained. The chimeric CD8A is expressed under the endogenous mouse Cd8a promoter, while native mouse Cd8a transcription and translation are disrupted.
Similarly, exons 1–3 and part of exon 4 of the mouse Cd8b1 gene—encoding the signal peptide and extracellular domain—are replaced by their human counterparts. Mouse regions encoding the transmembrane and cytoplasmic domains, as well as the promoter, 5'UTR, and 3'UTR, are preserved. The chimeric CD8B1 is driven by the endogenous mouse Cd8a promoter, and native mouse Cd8b1 gene transcription and translation are disrupted.
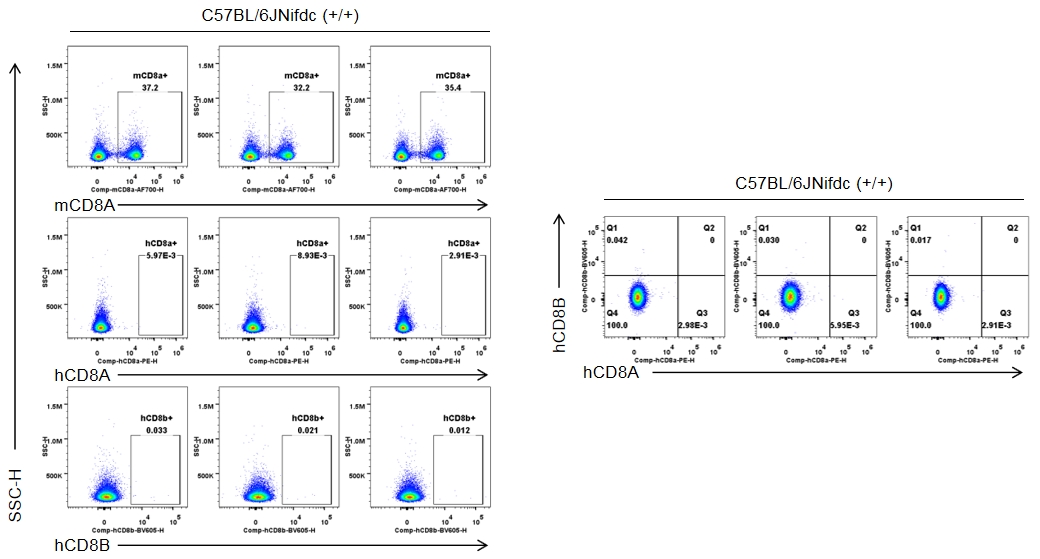
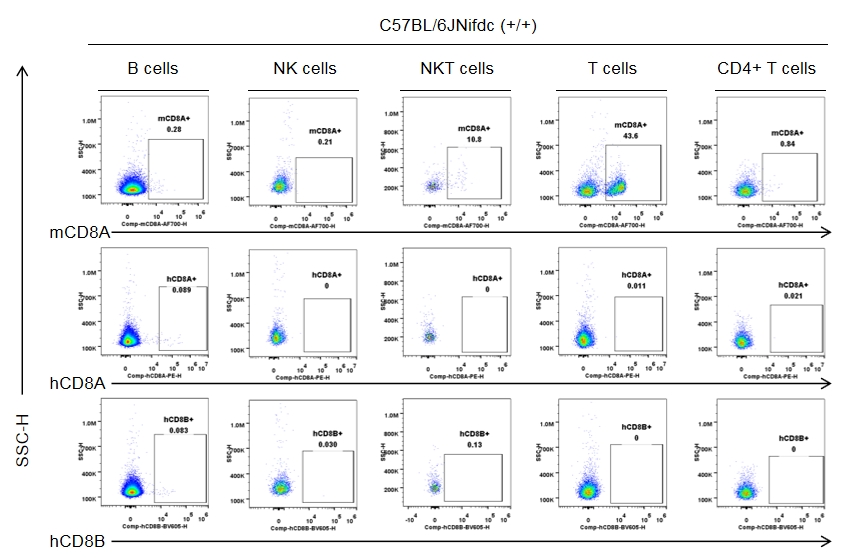
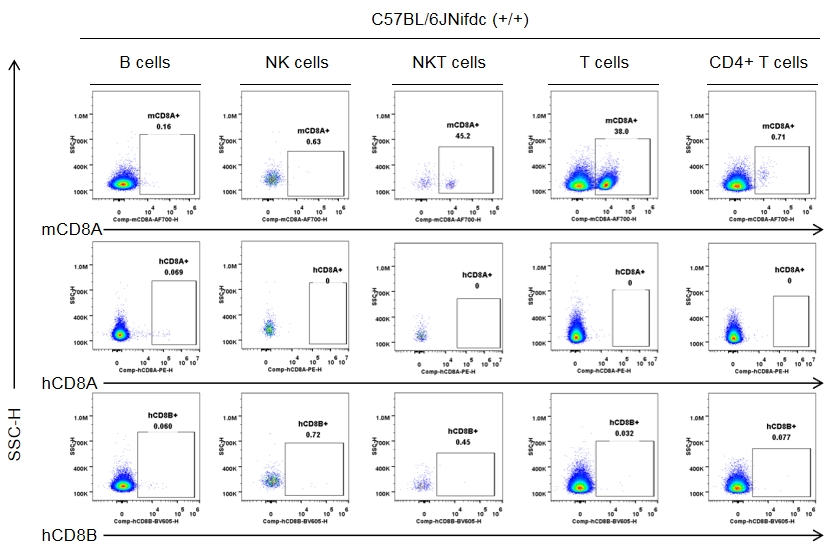
Strain-specific CD8 expression was analyzed in homozygous CD8 humanized mice by flow cytometry. Splenocytes were collected from wild-type C57BL/6JNifdc mice (+/+) and homozygous CD8 humanized mice (H/H), and analyzed using species-specific anti-CD8 antibodies (anti-human CD8A, Biolegend, 300908; anti-mouse CD8A, Biolegend, 100730; anti-human CD8B, BD, 742392). Mouse CD8A was detectable in wild-type mice.
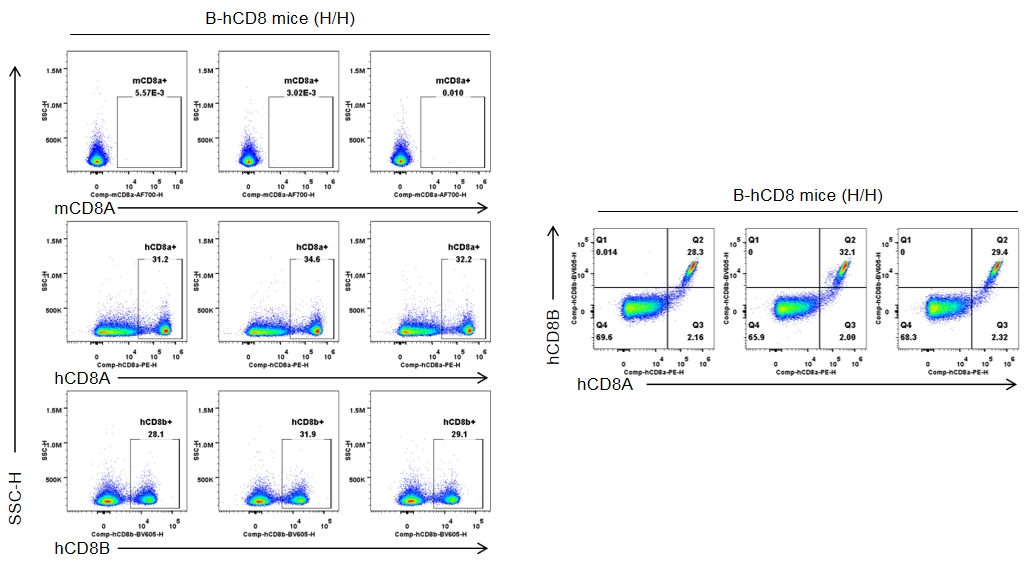
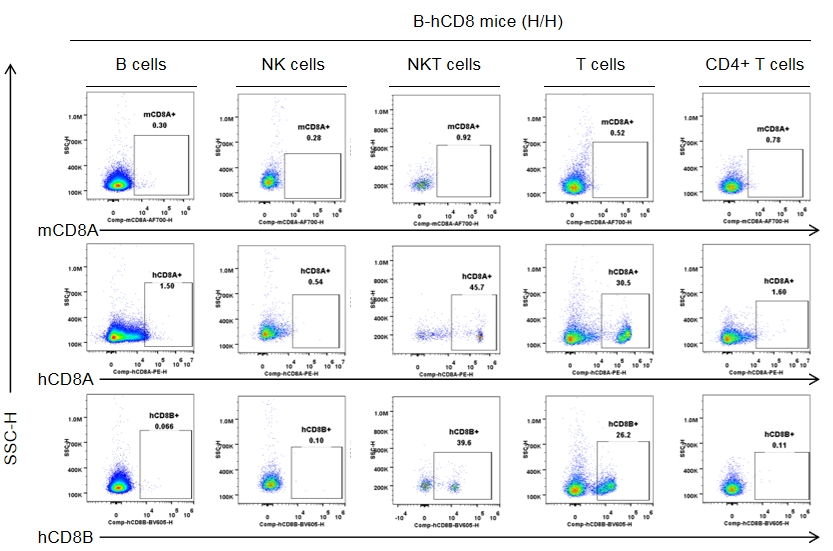
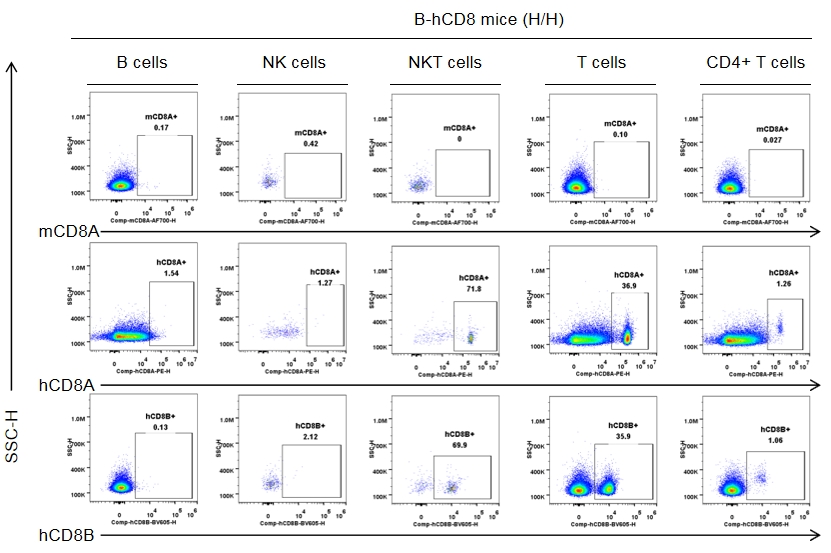
Strain-specific CD8 expression was analyzed in homozygous CD8 humanized mice by flow cytometry. Splenocytes were collected from wild-type C57BL/6JNifdc mice (+/+) and homozygous CD8 humanized mice (H/H), and analyzed using species-specific anti-CD8 antibodies (anti-human CD8A, Biolegend, 300908; anti-mouse CD8A, Biolegend, 100730; anti-human CD8B, BD, 742392). Human CD8A and human CD8B were exclusively detectable in homozygous CD8 humanized mice but not in wild-type mice.
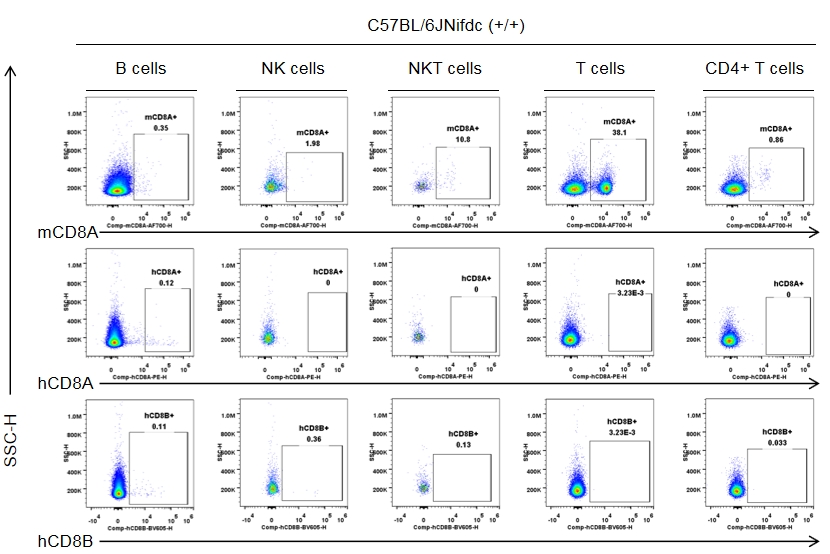
Strain specific CD8 expression analysis in wild-type C57BL/6JNifdc by flow cytometry. Splenocytes were collected from wild-type C57BL/6JNifdc mice (+/+, female, n=3, 9-week-old), and analyzed by flow cytometry with species-specific anti-CD8 antibody (anti-human CD8A, Biolegend, 300908; anti-mouse CD8A, Biolegend, 100730; anti-human CD8B, BD, 742392). Mouse CD8A was detectable in NKT cells and T cells from wild-type mice.
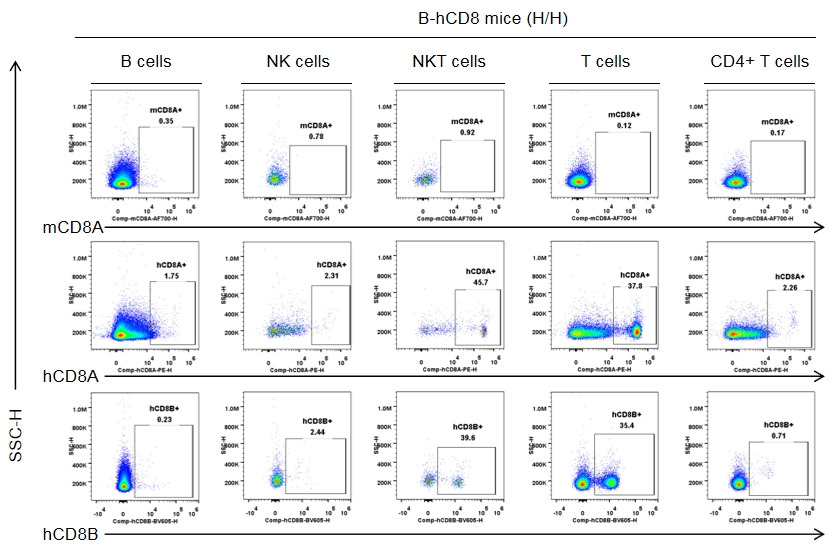
Strain specific CD8 expression analysis in homozygous CD8 humanized mice by flow cytometry. Splenocytes were collected from homozygous CD8 humanized mice (H/H, female, n = 3, 9-week-old), and analyzed by flow cytometry with species-specific anti-CD8 antibodies (anti-human CD8A, Biolegend, 300908; anti-mouse CD8A, Biolegend, 100730; anti-human CD8B, BD, 742392). Human CD8A and CD8B were detectable in NKT cells and T cells from CD8 humanized mice, but not in wild-type mice.
Strain specific CD8 expression analysis in wild-type C57BL/6JNifdc by flow cytometry. Blood cells were collected from wild-type C57BL/6JNifdc mice (+/+, female, n=3, 9-week-old), and analyzed by flow cytometry with species-specific anti-CD8 antibody (anti-human CD8A, Biolegend, 300908; anti-mouse CD8A, Biolegend, 100730; anti-human CD8B, BD, 742392). Mouse CD8A was detectable in NKT cells and T cells from wild-type mice.
Strain specific CD8 expression analysis in homozygous CD8 humanized mice by flow cytometry. Blood cells were collected from homozygous CD8 humanized mice (H/H, female, n = 3, 9-week-old), and analyzed by flow cytometry using species-specific anti-CD8 antibodies (anti-human CD8A, Biolegend, 300908; anti-mouse CD8A, Biolegend, 100730; anti-human CD8B, BD, 742392). Human CD8A and CD8B were detectable in NKT cells and T cells from CD8 humanized mice, but not in wild-type mice.
Strain specific CD8 expression analysis in wild-type C57BL/6JNifdc by flow cytometry. The lymph nodes were collected from wild-type C57BL/6JNifdc mice (+/+, female, n=3, 9-week-old), and analyzed by flow cytometry with species-specific anti-CD8 antibody (anti-human CD8A, Biolegend, 300908; anti-mouse CD8A, Biolegend, 100730; anti-human CD8B, BD, 742392). Mouse CD8A was detectable in NKT cells and T cells from wild-type mice.
Strain specific CD8 expression analysis in homozygous CD8 humanized mice by flow cytometry. The lymph nodes were collected from homozygous CD8 humanized mice (H/H, female, n = 3, 9-week-old) and analyzed using species-specific anti-CD8 antibodies (anti-human CD8A, Biolegend, 300908; anti-mouse CD8A, Biolegend, 100730; anti-human CD8B, BD, 742392). Human CD8A and CD8B were detectable in NKT cells and T cells from CD8 humanized mice, but not in wild-type mice.
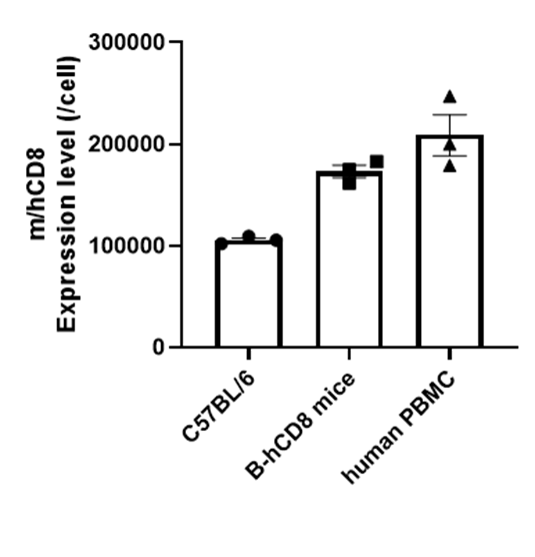
Strain specific CD8 quantitative expression analysis in homozygous B-hCD8 mice by flow cytometry. The blood which collected from wild-type C57BL/6 mice (+/+), homozygous B-hCD8 mice (H/H, female, n=3, 8-week-old) and human PBMC were analyzed by Quantum™ Simply Cellular® (Bangs Labs, 815 & 817) with species-specific anti-CD8 antibody (anti-human CD8A, Biolegend, 300908; anti-mouse CD8A, Biolegend, 100708). The CD8 quantitative expression on CD8+ T cells in the peripheral blood of B-hCD8 mice are closer to those observed in human PBMC than wild-type C57BL/6 mice.
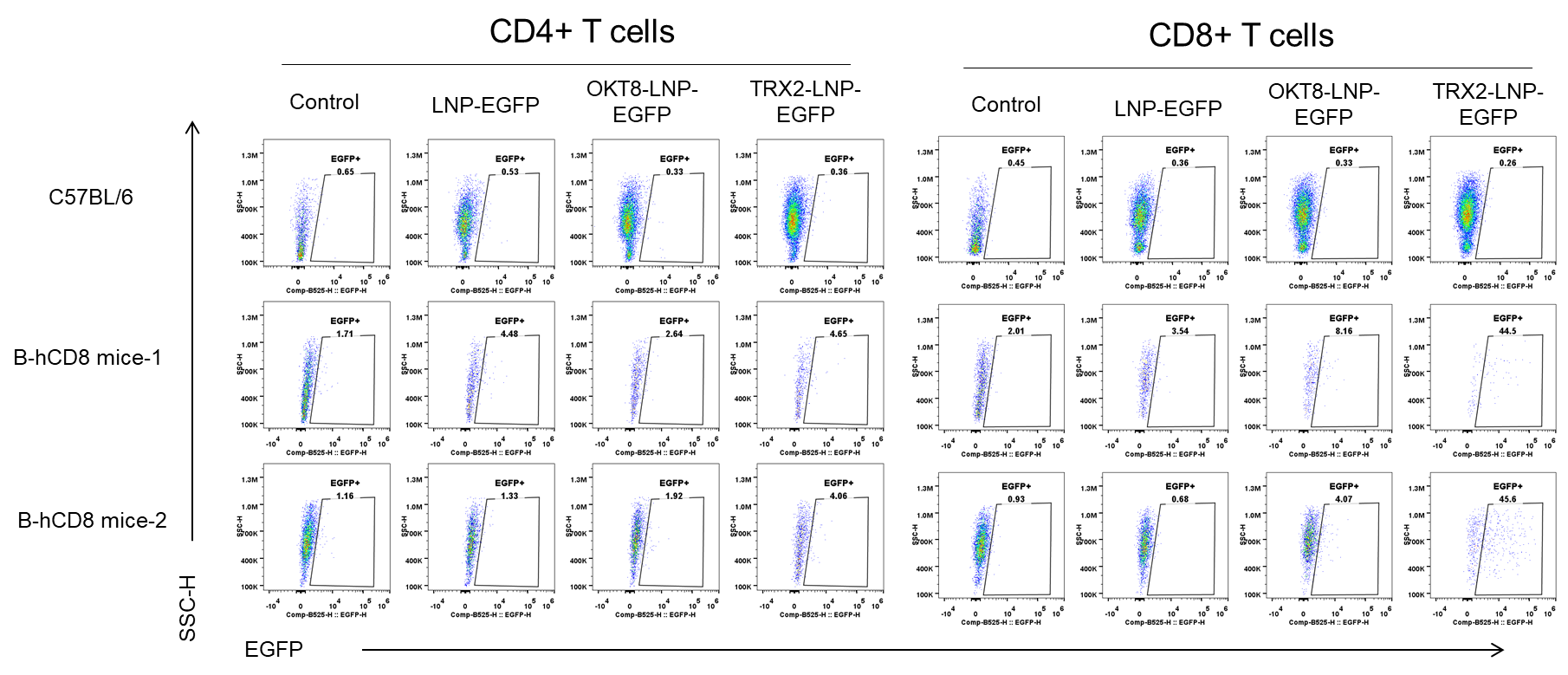
The delivery efficiency analysis of CD8-LNPs to CD8+ T cells in B-hCD8 mice in vitro. The T cells were isolated from spleens of C57BL/6 (+/+) and homozygous B-hCD8 mice (H/H, female, n=2, 9-week-old) using the pan T cell isolation kit (Miltenyi, 130-095-130), stimulated with anti-mCD3 (2μg/mL)and anti-mCD28 (5μg/mL) for 48 hours. The activated T cells were treated with different LNP-EGFP formulations (in house) for 24h. The EGFP was detectable in CD8+ T cells from B-hCD8 mice, but not in the wild-type mice.
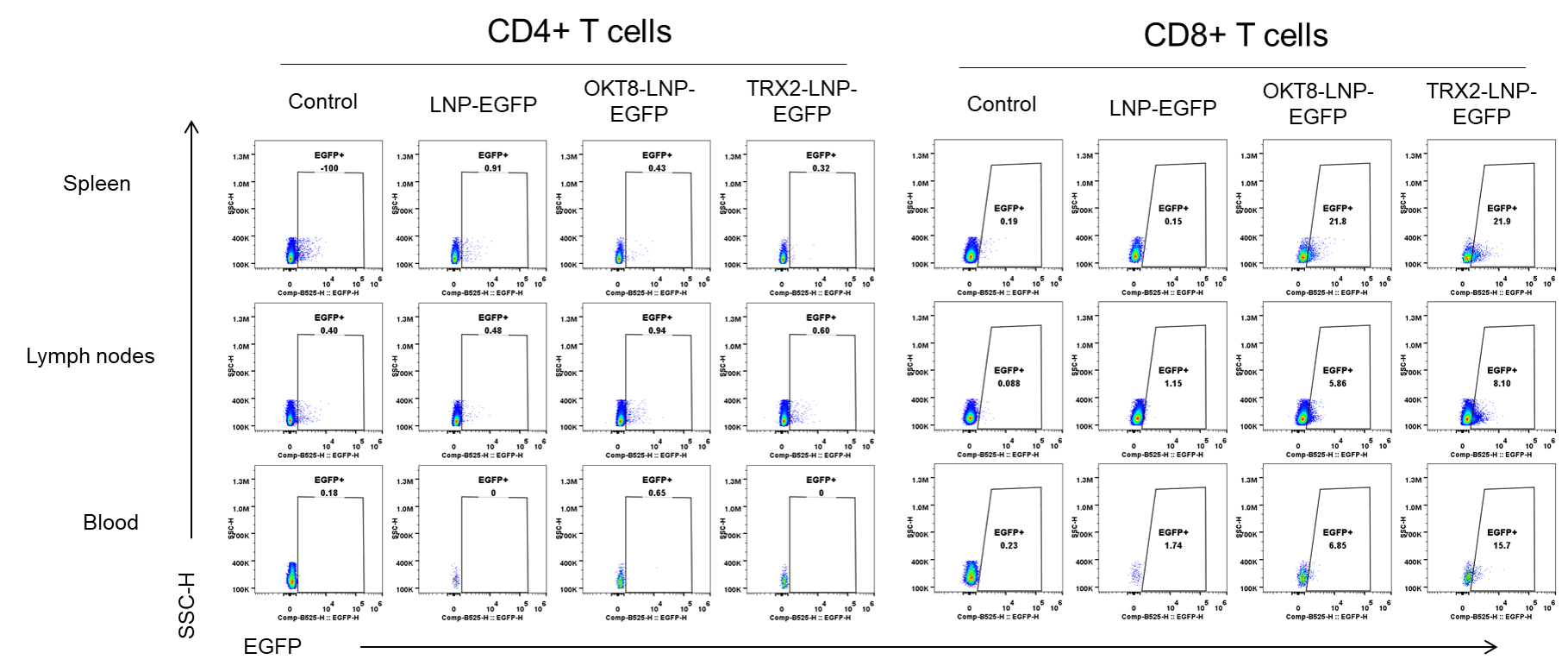
The delivery efficiency analysis of CD8-LNPs to CD8+ T cells in B-hCD8 mice in vivo. Spleens, lymph nodes and blood were collected from C57BL/6 (+/+) and homozygous B-hCD8 mice (H/H, female, n=2, 14-week-old), which treated with different LNP-EGFP formulations (in house, 30μg/250μL, i.v.) for 24h. Then the EGFP was detectable in CD8+ T cells (not CD4+ T cells) from spleen, lymph nodes and blood of B-hCD8 mice, but not in the wild-type mice.
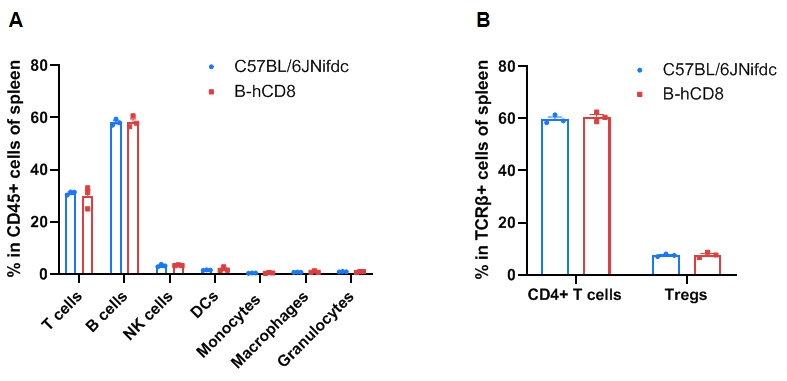
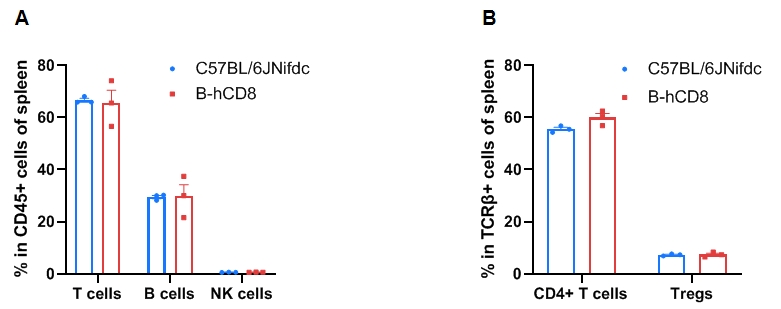
Leukocyte subpopulation frequencies in the spleen of CD8 humanized mice. Splenocytes were isolated from wild-type C57BL/6JNifdc mice and homozygous CD8 humanized mice (female, 9-week-old, n = 3). (A) Flow cytometry analysis was performed to assess the frequency of leukocyte subpopulations. (B) Frequency of T-cell subpopulations. Frequencies of T cells, B cells, NK cells, dendritic cells (DCs), granulocytes, monocytes, macrophages, CD4⁺ T cells, and Tregs in CD8 humanized mice were similar to those in C57BL/6 mice, demonstrating that humanization of CD8 does not alter the frequency or distribution of these cell types in the spleen. Values are expressed as mean ± SEM. Significance was determined by two-way ANOVA. *P < 0.05, **P < 0.01, ***P < 0.001.
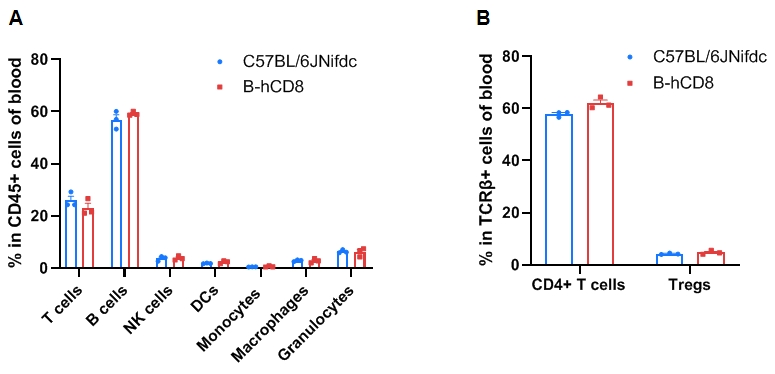
Leukocyte subpopulation frequencies in the blood of CD8 humanized mice. Blood cells were isolated from wild-type C57BL/6JNifdc mice (female, 9-week-old, n = 3) and homozygous CD8 humanized mice (female, 9-week-old, n = 3). (A) Flow cytometry analysis was performed to assess the frequency of leukocyte subpopulations. (B) Frequency of T-cell subpopulations. Frequencies of T cells, B cells, NK cells, dendritic cells (DCs), granulocytes, monocytes, macrophages, CD4⁺ T cells, and Tregs in CD8 humanized mice were similar to those in C57BL/6JNifdc mice, indicating that CD8 humanization does not change immune-cell distribution in the blood. Values are expressed as mean ± SEM. Significance was determined by two-way ANOVA. *P < 0.05, **P < 0.01, ***P < 0.001.
Leukocyte subpopulation frequencies in the lymph nodes of CD8 humanized mice. Lymph-node cells were isolated from wild-type C57BL/6JNifdc mice (female, 9-week-old, n = 3) and homozygous CD8 humanized mice (female, 9-week-old, n = 3). (A) Flow cytometry analysis was performed to assess the frequency of leukocyte subpopulations. (B) Frequency of T-cell subpopulations. Frequencies of T cells, B cells, NK cells, CD4⁺ T cells, and Tregs in CD8 humanized mice were similar to those in C57BL/6 mice, demonstrating that CD8 humanization does not alter cell-type frequencies in the lymph nodes. Values are expressed as mean ± SEM. Significance was determined by two-way ANOVA. *P < 0.05, **P < 0.01, ***P < 0.001.
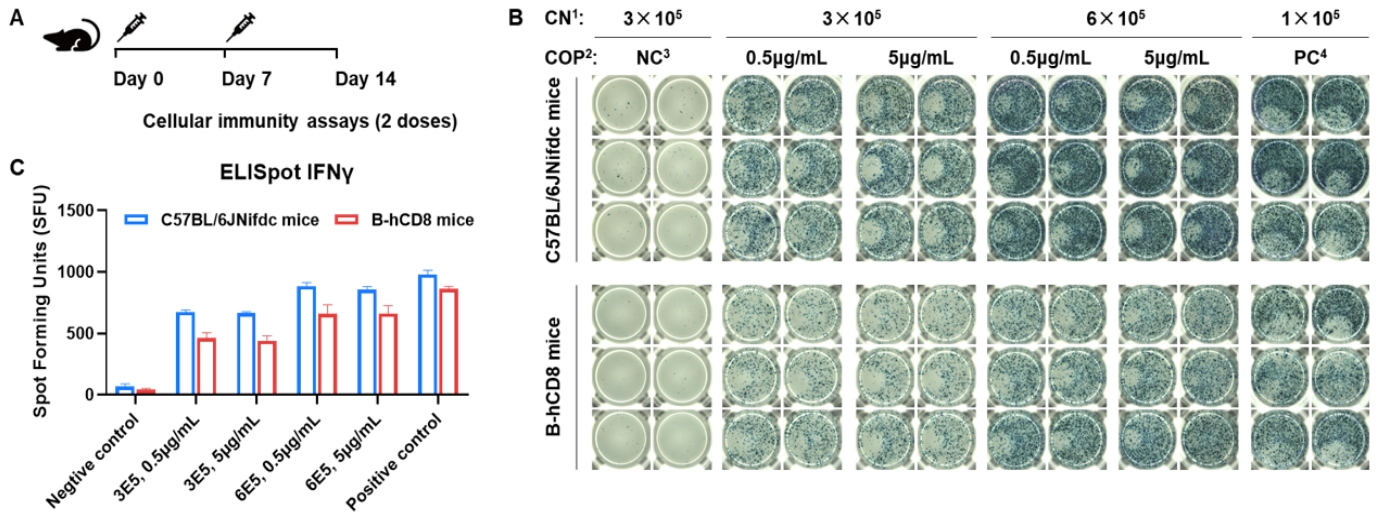
Detection of OVA-induced immune responses in CD8 humanized mice by IFN-γ ELISpot assay. (A) Scheme of OVA immunization and testing. Female wild-type C57BL/6JNifdc mice and CD8 humanized mice at 9–10 weeks of age were immunized by intraperitoneal injection of 0.5 mg OVA protein (Sigma, A5503-25MG) and 50 μg poly(I:C) (InvivoGen, tlrl-pic). Mice were immunized twice at a 1-week interval. One week after the final immunization, mice were sacrificed. Splenocytes were extracted and stimulated with OVA peptide 257–264, no peptide as a negative control (NC), or Cell Activation Cocktail (without Brefeldin A; Biolegend, 42330) as a positive control, followed by measurement of IFN-γ secretion. No significant difference in body weight was observed among groups (data not shown). (B) Representative results show splenocyte stimulation with negative control, OVA peptide 257–264, or positive control in duplicates. (C) Summary of results. These data indicate that CD8 humanized mice have normal T-cell immunogenic function. 1, CN: Cell number. 2, COP: Concentration of the peptide. 3, NC: Negative control. 4, PC: Positive control.
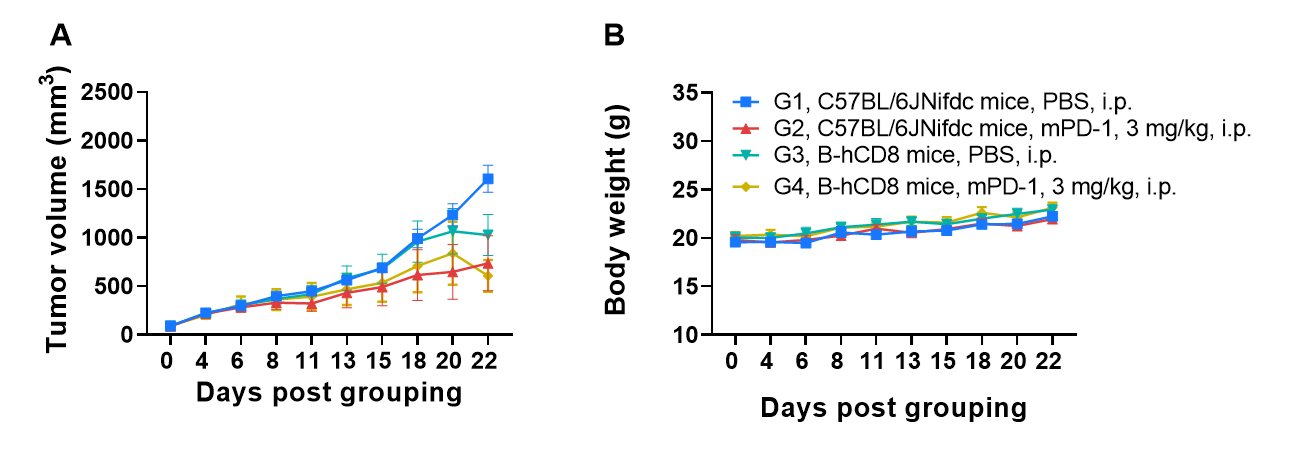
Comparison of anti-PD-1 antibody efficacy between C57BL/6JNifdc mice and B-hCD8 mice. (A) Effect of anti-mouse PD-1 antibody on MC38 tumor growth in C57BL/6JNifdc mice and B-hCD8 mice. Treatment with PBS or anti-mouse PD-1 antibody when tumor volume reached approximately 100 mm3. As shown in panel A, anti-mPD-1 antibody treatment exhibited stronger inhibitory efficacy in both C57BL/6JNifdc mice and B-hCD8 mice. (B) Body weight changes during tumor growth observation. These results demonstrate that B-hCD8 mice exhibited anti-tumor efficacy comparable to wild-type C57BL/6JNifdc mice, confirming its functional competence in mediating tumor cell killing. Values are expressed as mean ± SEM.
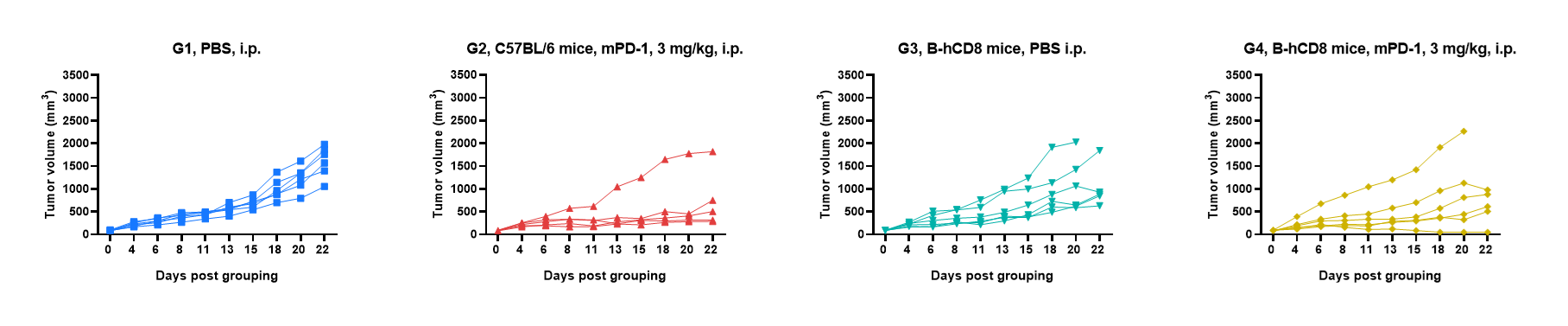
Antitumor activity of anti-mouse PD-1 antibody against syngeneic tumors. MC38 tumor cells growth of individual mice.
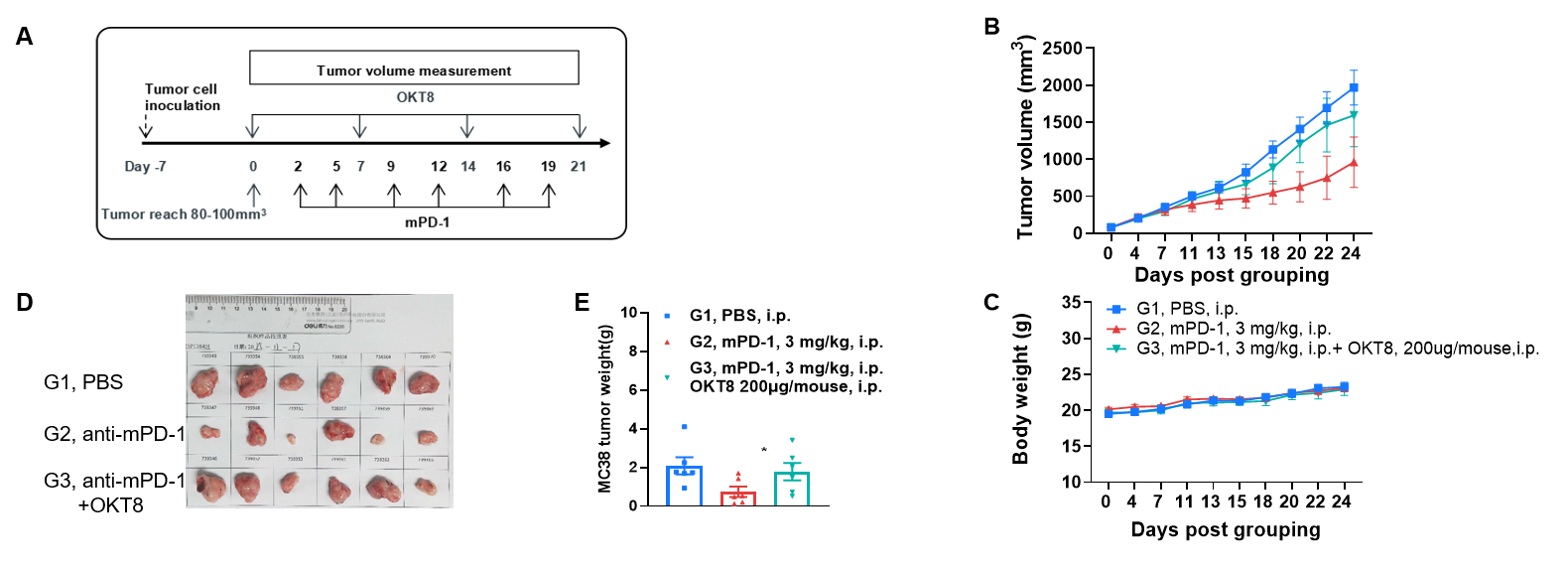
The impact of CD8 + T cell depletion on the efficacy of anti-mouse PD-1 antibody (A) Experimental scheme. (B) Effect of anti-mouse PD-1 antibody on MC38 tumor growth in B-hCD8 mice, with or without CD8+ T cell depletion. (C) Body weight changes during tumor growth observation. (D-E) MC38 tumor weight measurement after the mice were sacrificed. B-hCD8 mice received a subcutaneous flank injection of 5×105 MC38 cells. Treatment with anti-mouse PD-1 antibody or the CD8+ T cell-depleting antibody OKT8 was initiated when tumor volume reached approximately 100 mm3, following the regimen detailed in the pane A. As shown in panel B, anti-mPD-1 antibody treatment exhibited stronger inhibitory efficacy than CD8+ T cell depletion, demonstrating the critical tumor-killing function of CD8+ T cells in B-hCD8 mice. These results validate the B-hCD8 mice as a powerful preclinical model for in vivo assessment of anti-tumor efficacy in the context of a humanized CD8+ T cell compartment. Values are expressed as mean ± SEM.
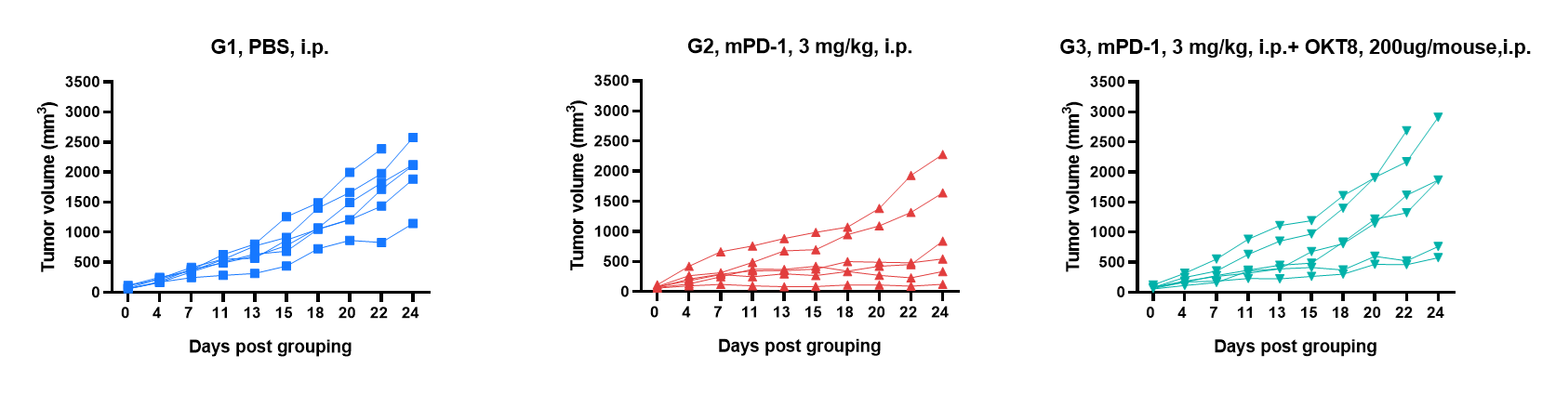
Antitumor activity of anti-mouse PD-1 antibody against syngeneic tumors. MC38 tumor cells growth of individual mice.
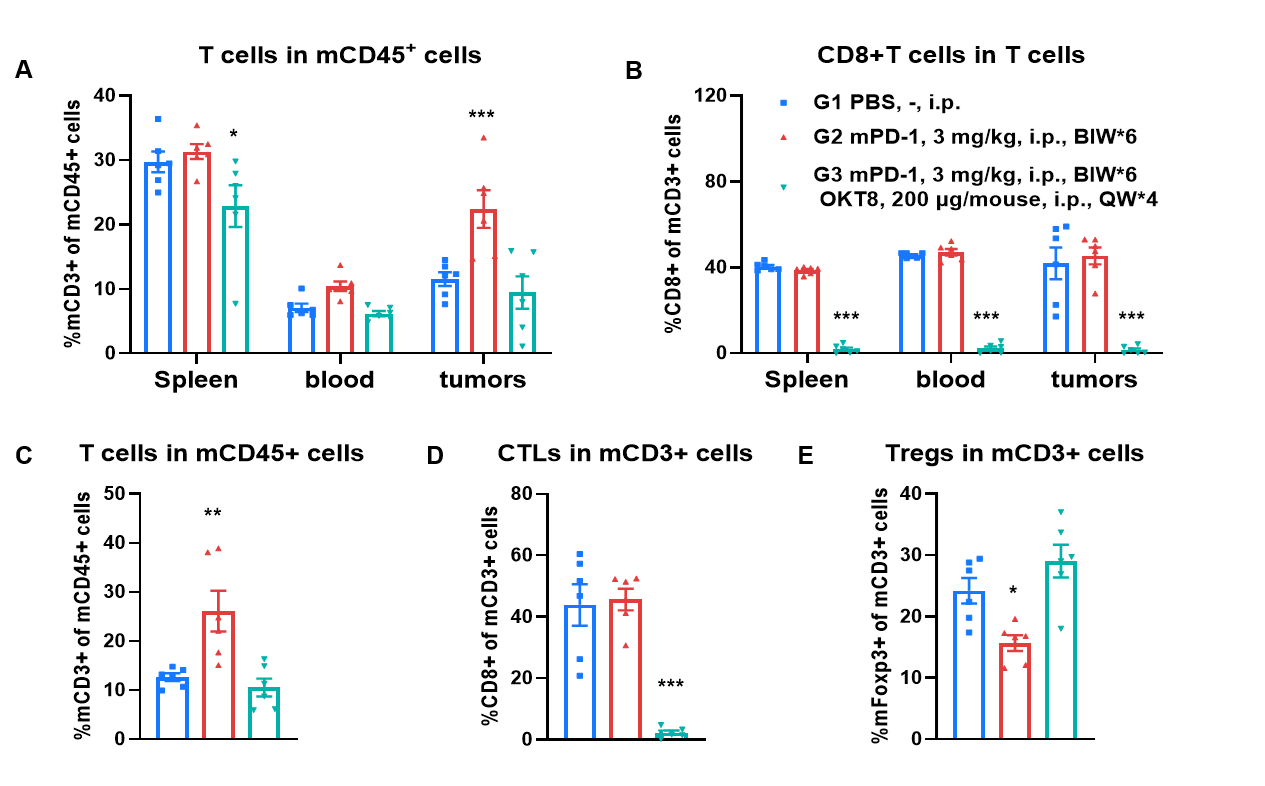
The impact of CD8 + T cell depletion on the efficacy of anti-mouse PD-1 antibody. (A-B) Demonstration of CD8+ T cell depletion by flow cytometry. Depletion was performed by OKT8 administration once per week (200 μg/mouse) starting 2 days prior to start of treatments. There was a significant decreasing of CD3+ T cells in the spleen, whereas no significance was observed in the blood and tumors for the combination of anti-mPD-1 antibody and OKT8 (A). Demonstration of near complete CD8+ T cell depletion in spleen, blood and tumor on day 24 from last OKT8 administration in combination of anti-mPD-1 antibody and OKT8 (B). (C-E) Tumors from MC38 tumor-bearing mice that were treatmented with the PBS, anti-mPD-1, and combination of anti-mPD-1 antibody and OKT8 were analyzed on day 24. Analysis of CD3+ T, CD8+ T and Tregs in the tumors determined by the flow cytometric assay. For tumor-infiltrated T (C) and Tregs (E), the percentages (in CD45+ cells) were not significantly changed, tumor-infiltrated CD8+ T cells was decreased significantly in combination of anti-mPD-1 antibody and OKT8 (D).
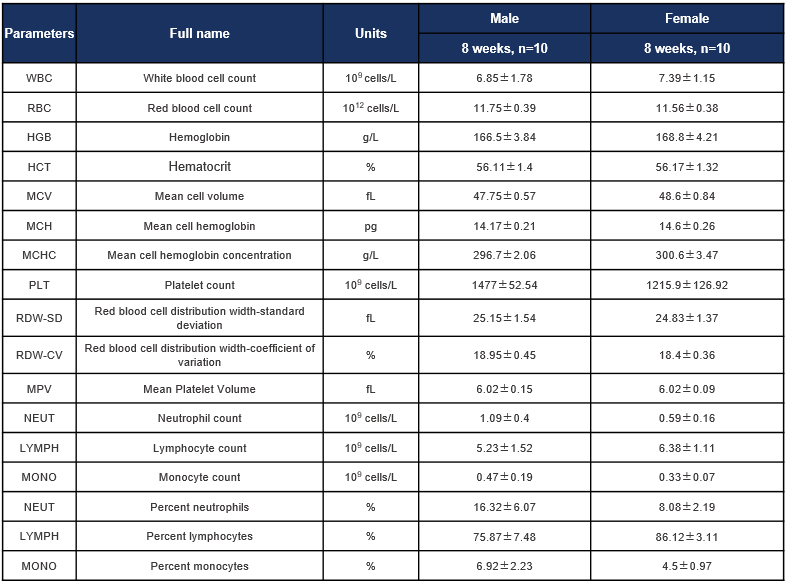
Complete blood count (CBC) of C57BL/6JNifdc mice. Values are expressed as mean ± SD.
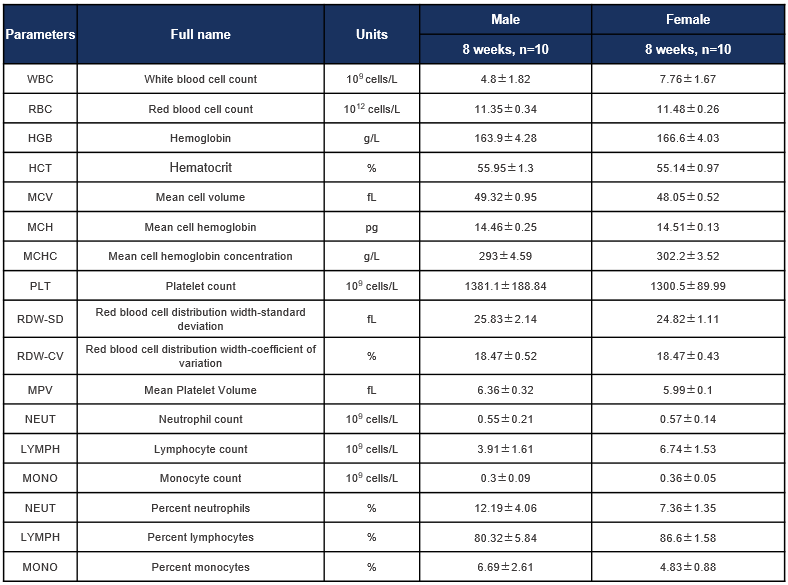
Complete blood count (CBC) of B-hCD8 mice. Values are expressed as mean ± SD.
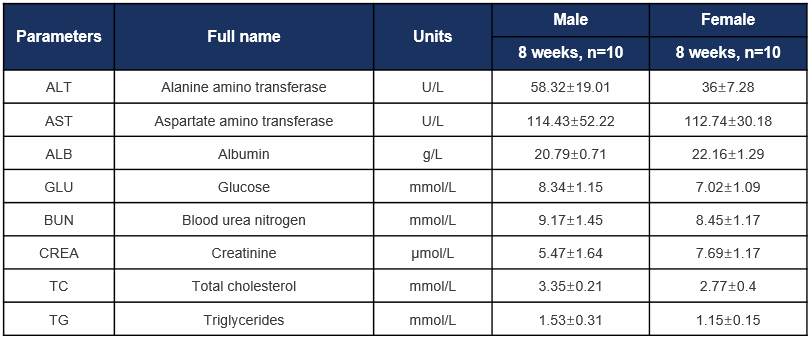
Biochemical test of C57BL/6JNifdc mice. Values are expressed as mean ± SD.
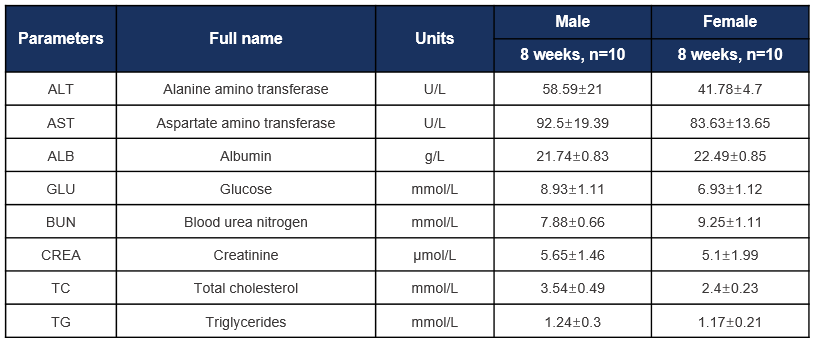
Biochemical test of B-hCD8 mice. Values are expressed as mean ± SD.
The organs of female C57BL/6JNifdc mice (8-week-old, n=10).
The organs of male C57BL/6JNifdc mice (8-week-old, n=10).
The organs of female B-hCD8 mice (8-week-old, n=10).
The organs of male B-hCD8 mice (8-week-old, n=10).
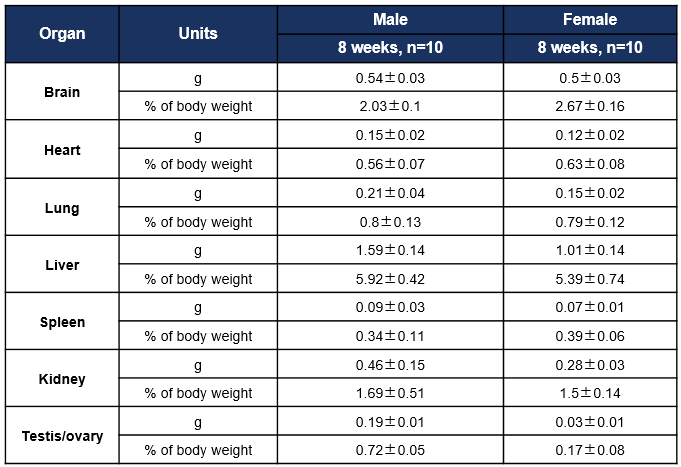
Average weight of the main organs of C57BL/6JNifdc mice. Values are expressed as mean ± SD.
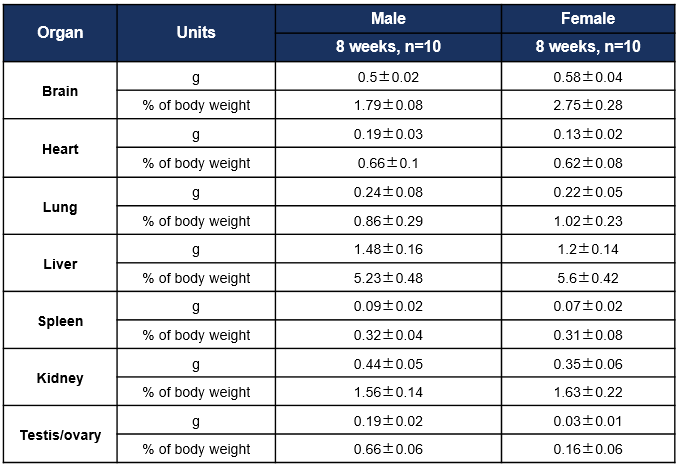
Average weight of the main organs of B-hCD8 mice. Values are expressed as mean ± SD.
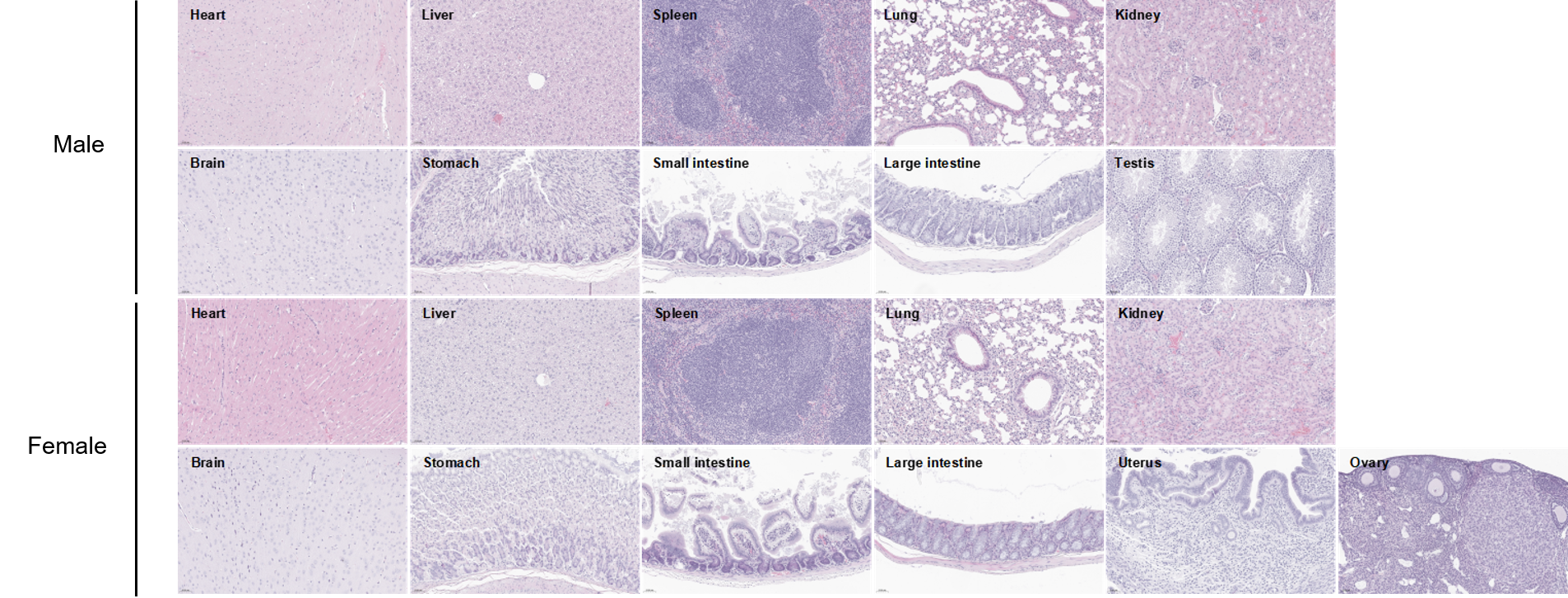
Histopathological analysis of organs in C57BL/6JNifdc mice. The main organs of C57BL/6JNifdc mice were isolated at 8 weeks of age and analyzed with H&E staining (male, n=10; female, n=10). Results showed that no obvious abnormalities were found in all of the organs (heart, liver, spleen, lung, kidney, brain, stomach, small intestine, large intestine, ovary, uterus and testis). Scale bar: 50 μm.
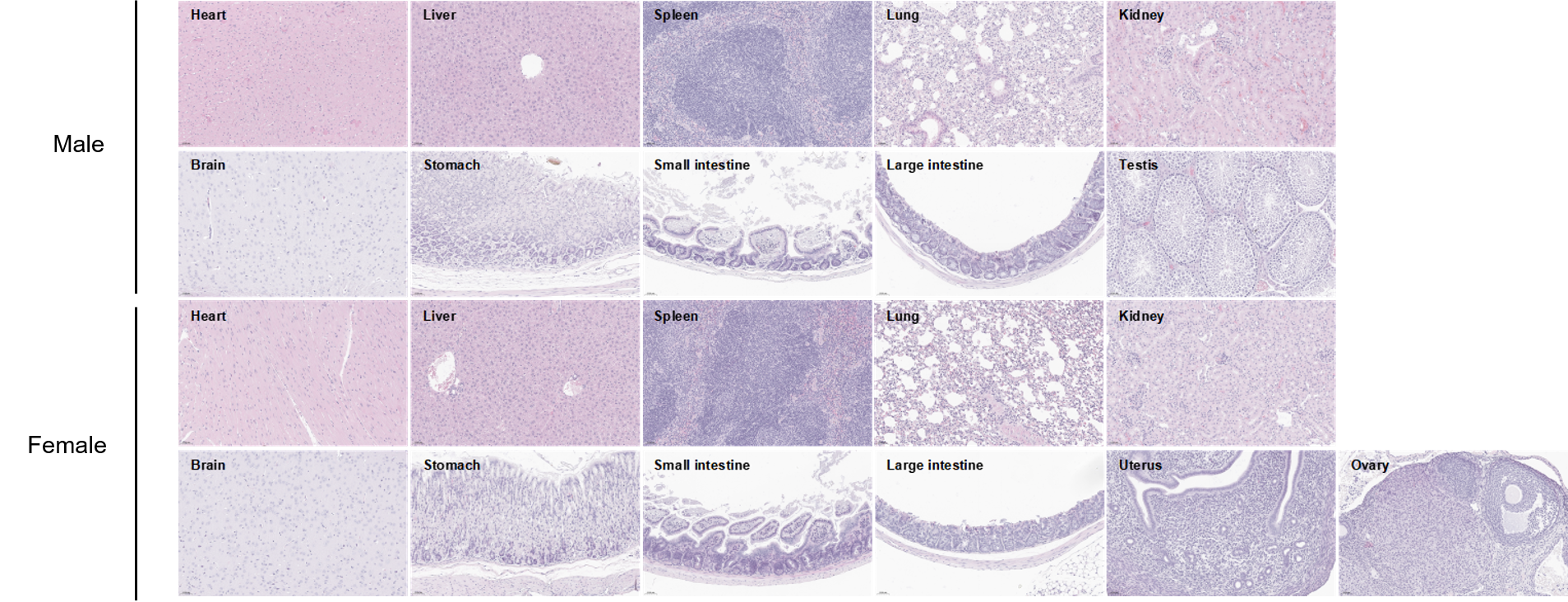
Histopathological analysis of organs in B-hCD8 mice. The main organs of B-hCD8 mice were isolated at 8 weeks of age and analyzed with H&E staining (male, n=10; female, n=10). Results showed that no obvious abnormalities were found in all of the organs (heart, liver, spleen, lung, kidney, brain, stomach, small intestine, large intestine, ovary, uterus and testis). Scale bar: 50 μm.
Q1: What are humanized CD8 mice used for?
B-hCD8 mice express human CD8A/CD8B on cytotoxic T cells, making them suitable for human CD8-targeting antibody validation, preclinical immuno-oncology studies, and T-cell activation research.
Q2: Do B-hCD8 mice maintain normal immune cell composition?
Yes. Spleen, blood, and lymph-node data (n=3, female, 9 weeks) show T cells, B cells, NK cells, dendritic cells, granulocytes and monocytes remain comparable to WT controls.
Q3: Can B-hCD8 mice be used for in vivo antibody efficacy testing?
Absolutely. The physiologic human CD8 expression enables in vivo binding, depletion, activation, and mechanistic studies for human-specific CD8 antibodies.
Q4: Do B-hCD8 mice mount normal T-cell responses?
Yes. OVA immunization experiments confirm intact antigen-specific T-cell immunity.
Q5: Are these mice on a widely used genetic background?
Yes. They are on the C57BL/6 background, compatible with tumor models, IO studies, adoptive transfer, vaccine studies, and combination-therapy designs.