
NOD.CB17-Prkdcscid Il2rgtm1Bcgen B2mtm1Bcgen Fcgrttm1(B2m/Fcgrt)Bcgen /Bcgen • 110601

| Product name | B-NDG B2m KO mice plus |
|---|---|
| Catalog number | 110601 |
| Strain name | NOD.CB17-Prkdcscid Il2rgtm1Bcgen B2mtm1Bcgen Fcgrttm1(B2m/Fcgrt)Bcgen /Bcgen |
| Strain background | B-NDG |
| NCBI gene ID | 12010,16186,19090,14132 (Mouse) |
| Aliases | Ly-m11; beta2m; beta2-m; gc; p64; [g]c; CD132; gamma(c); p460; scid; slip; DNAPK; DNPK1; HYRC1; XRCC7; dxnph; DOXNPH; DNAPDcs; DNA-PKcs; FcRn |
| Application | Reduce PBMC reconstitution GVHD response and prolong experimental window |
Human PBMC-engrafted mouse models are widely used for evaluating human immune responses in vivo. While human PBMCs can successfully engraft in B-NDG mice, the experimental window is limited due to the rapid onset of xenogeneic GvHD driven by mismatches between human and mouse MHC molecules. Because MHC class I requires the β2-microglobulin (β2m) chain encoded by the B2m gene, loss of B2M disrupts surface expression of MHC-I and helps attenuate GvHD severity.
However, B2M is also essential for FcRn, the receptor responsible for maintaining IgG half-life. Conventional B2m knockout models therefore exhibit shortened antibody exposure.
To overcome this limitation, Biocytogen developed the B-NDG B2m KO mice plus, in which the mouse B2m gene is knocked out and replaced with a mouse B2m–Fcgrt fusion gene inserted at the Fcgrt locus. This model does not express murine MHC class I but retains normal IgG pharmacokinetics, delays GvHD onset, and provides a practical platform for evaluating xenogeneic GvHD, antitumor drug efficacy, and PBMC-based humanized immune responses in vivo.
Key Advantages
Validation
Applications
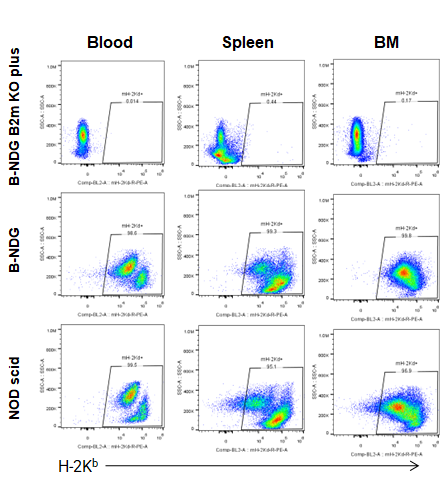
Blood, splenocytes, and bone marrow (BM) cells were collected from B-NDG B2m KO mice plus, B-NDG, and NOD-scid mice (n=5) and analyzed using anti–H-2Kd. MHC class I expression was detectable in B-NDG and NOD-scid mice but absent in B-NDG B2m KO mice plus.
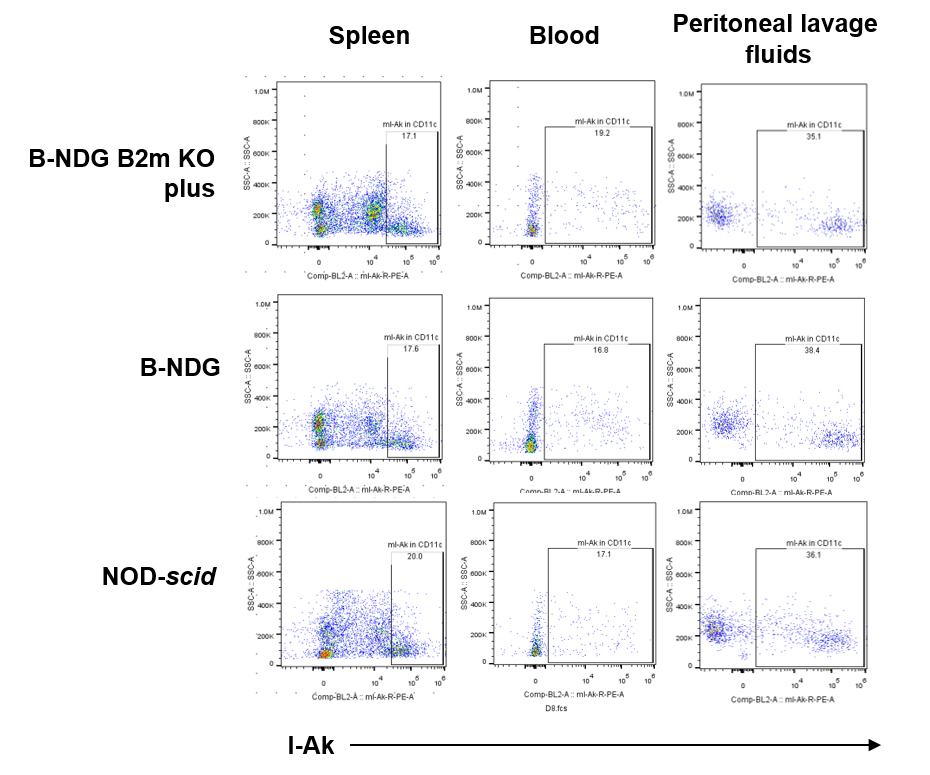
Blood, splenocytes, and peritoneal lavage cells were collected from B-NDG B2m KO mice plus, B-NDG, and NOD-scid mice (n=3). Single live CD45⁺CD11b⁺CD11c⁺ dendritic cells (DCs) were analyzed for MHC class II (mIA-IE). MHC-II expression was detectable across DC subpopulations in all three strains.
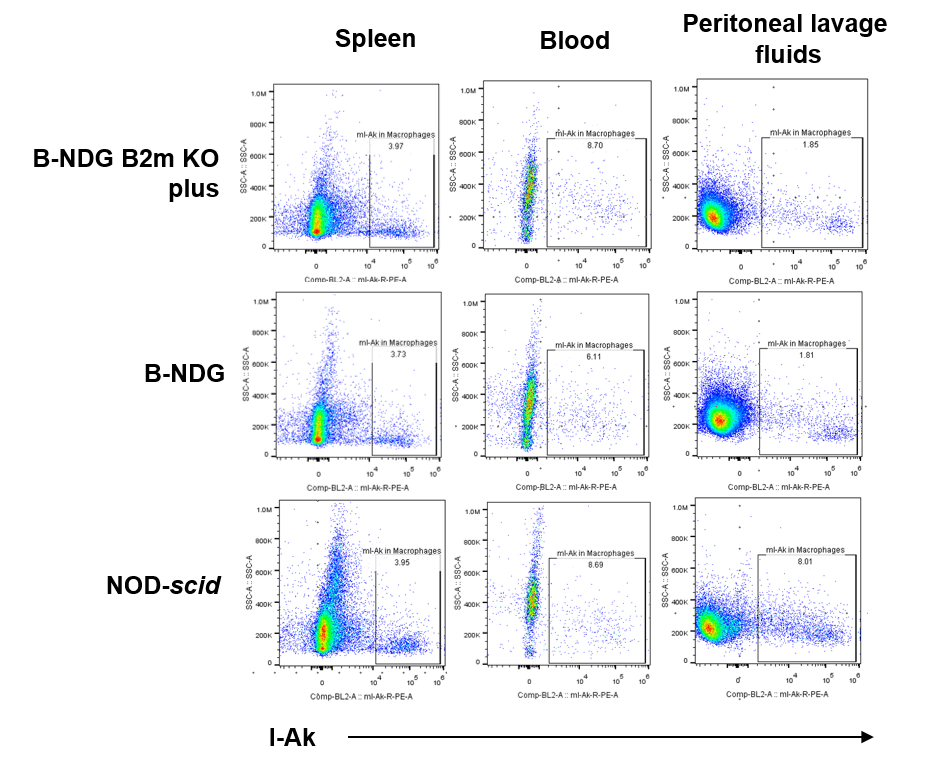
Blood, splenocytes, and peritoneal lavage samples were collected from B-NDG B2m KO Mice Plus, B-NDG, and NOD-scid mice (n=3). CD45⁺CD11b⁺F4/80⁺ macrophages from B-NDG B2m KO mice plus, B-NDG, and NOD-scid mice all expressed MHC-II.
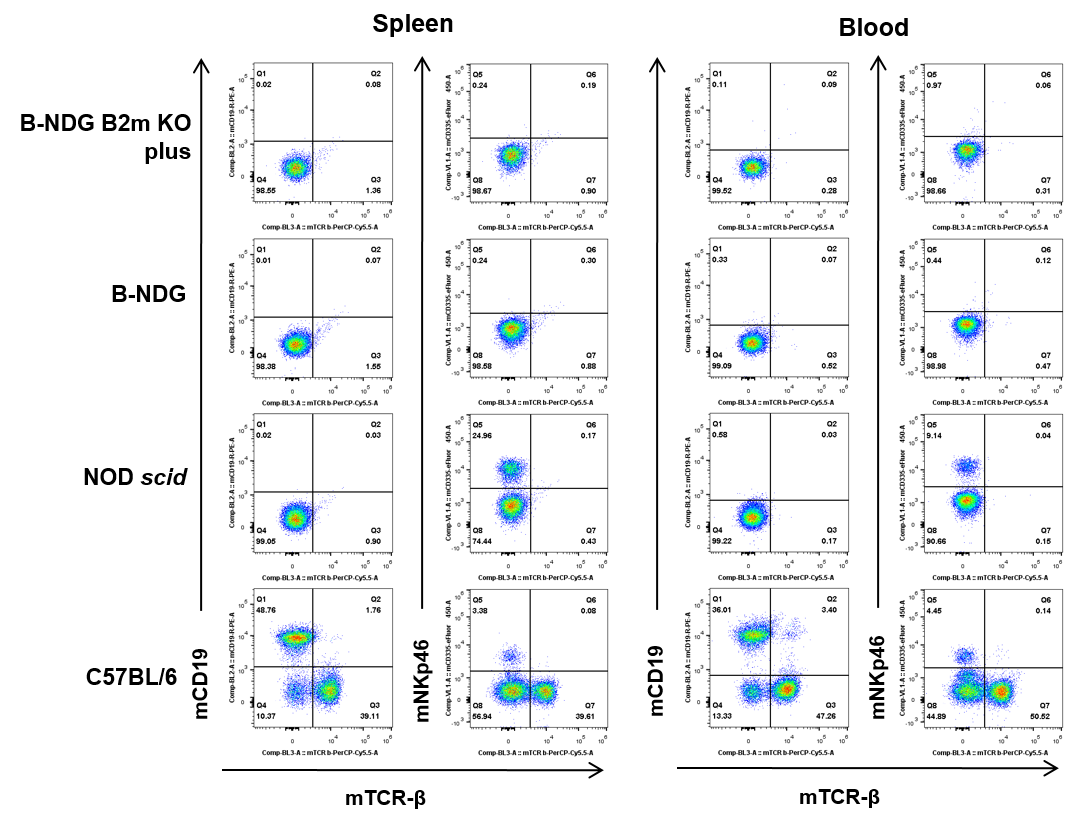
Blood and splenocytes were collected from B-NDG B2m KO mice plus, B-NDG, NOD-scid, and C57BL/6 mice (n=5). T, B, and NK cells were undetectable in both B-NDG B2m KO mice plus and B-NDG mice. T and B cells were absent, but NK cells were detectable in NOD-scid mice. All lymphocyte subsets were detectable in C57BL/6 mice.
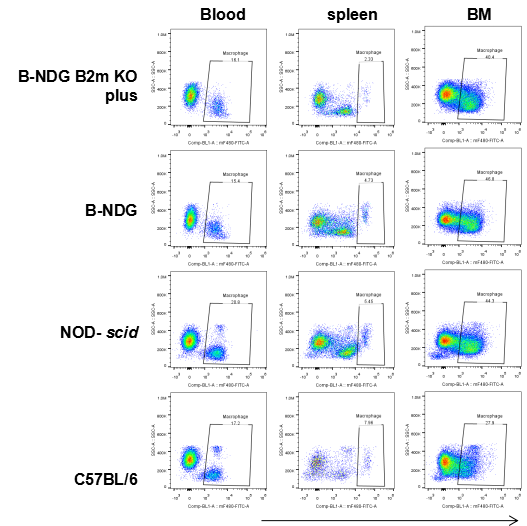
Blood, splenocytes, and bone marrow (BM) cells were collected from B-NDG B2m KO mice plus, B-NDG, NOD-scid, and C57BL/6 mice (n=5). Macrophages (mCD45⁺mCD11b⁺mF4/80⁺) were detectable in all tested strains, including B-NDG B2m KO mice plus.
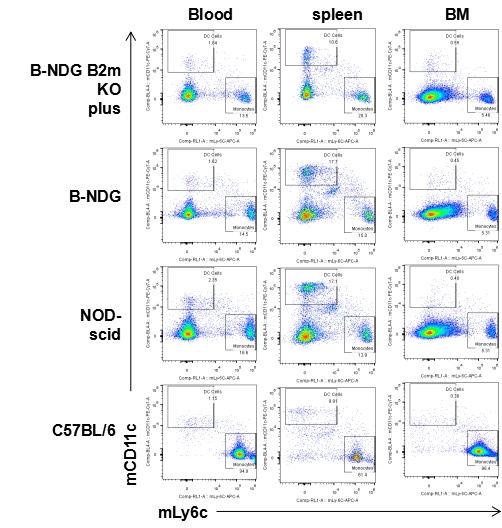
Blood, splenocytes, and bone marrow (BM) cells were collected from B-NDG B2m KO mice plus, B-NDG, NOD-scid, and C57BL/6 mice. Dendritic cells (mCD45⁺mCD11b⁺mCD11c⁺) and monocytes (mCD45⁺mCD11b⁺mLy6c⁺) were detectable in blood, spleen, and bone marrow of B-NDG B2m KO mice plus, B-NDG, NOD-scid, and C57BL/6 mice.
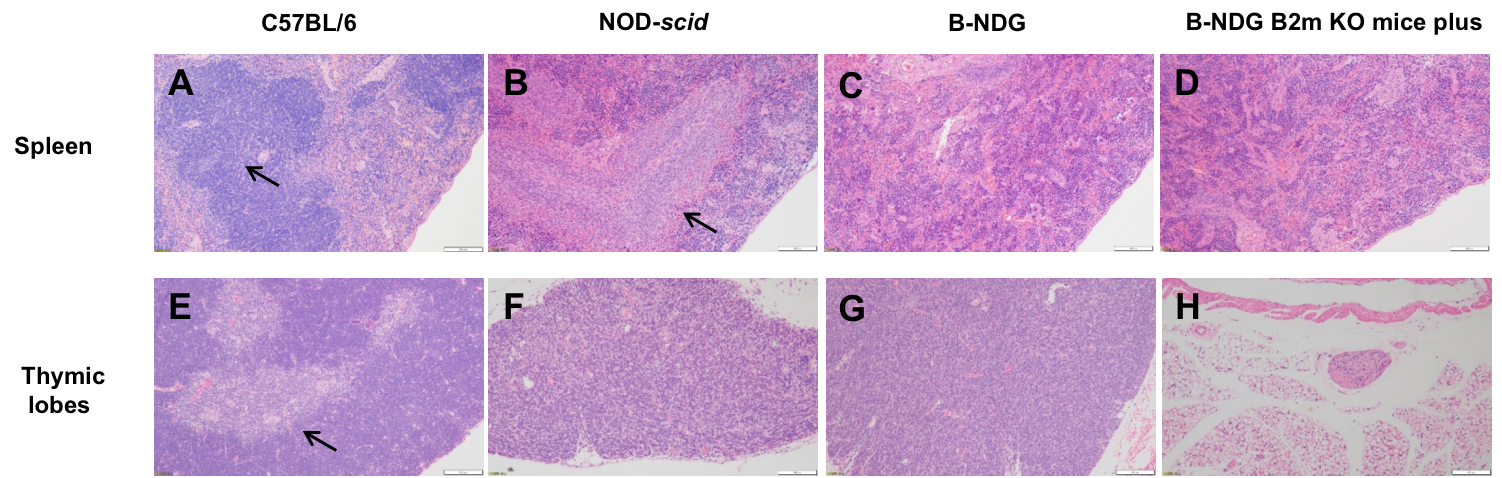
Histopathological sections of the spleen and thymus from B-NDG B2m KO Mice Plus, B-NDG, NOD-scid, and C57BL/6 mice were analyzed. (A) C57BL/6 spleen shows intact follicles. (B) NOD-scid spleen displays white-pulp hypoplasia. (C, D) B-NDG and B-NDG B2m KO mice plus spleens show complete follicular loss. (E) C57BL/6 thymus has a well-defined cortex. (F) NOD-scid thymus is hypoplastic without a defined cortex. (G) B-NDG thymus is severely hypoplastic. (H) B-NDG B2m KO mice plus show no visible thymic lobes in the normal anatomic position.
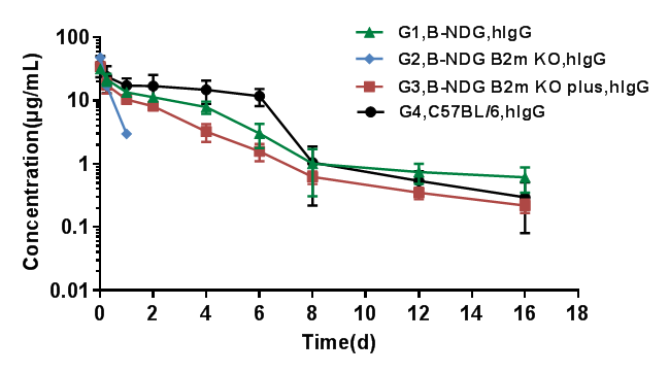
Pharmacokinetic characteristic of B-NDG B2m KO mice plus has no difference compared with wild-type mice.
Homozygous B-NDG, B-NDG B2m mice, B-NDG B2m KO mice plus and C57BL/6 mice were treated with human IgG (n=5). Blood samples were collected at different time point for the PK assay. The results showed that the PK results of B-NDG and B-NDG B2m KO mice plus groups were basically in line with the pharmacokinetic characteristics, with no difference compared with wild-type mice, and the drug concentration of B-NDG B2m KO mice group could not be measured at the time point 2 days later.
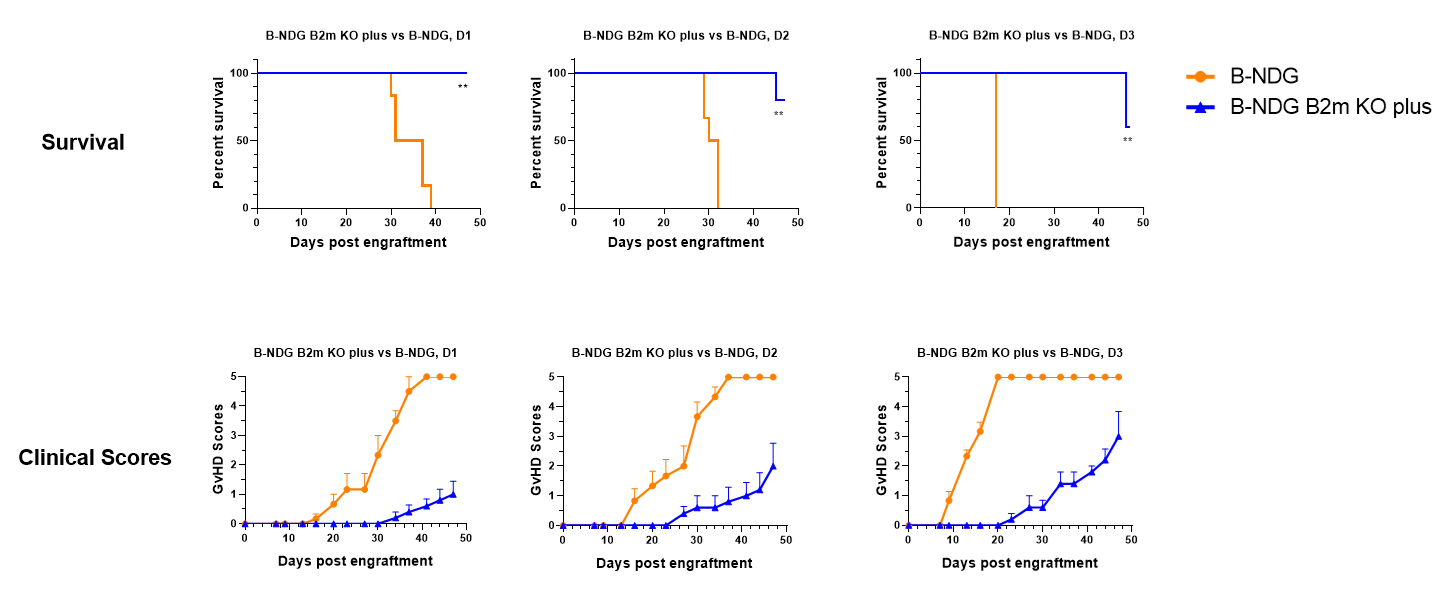
B-NDG B2m KO Mice Plus (11-week-old females, n=5) and B-NDG mice (10-week-old females, n=6) were engrafted with human PBMCs (2×10⁶) from three healthy donors. Clinical GvHD was scored twice weekly. B-NDG B2m KO mice plus showed significantly extended survival compared with B-NDG mice (P < 0.01).
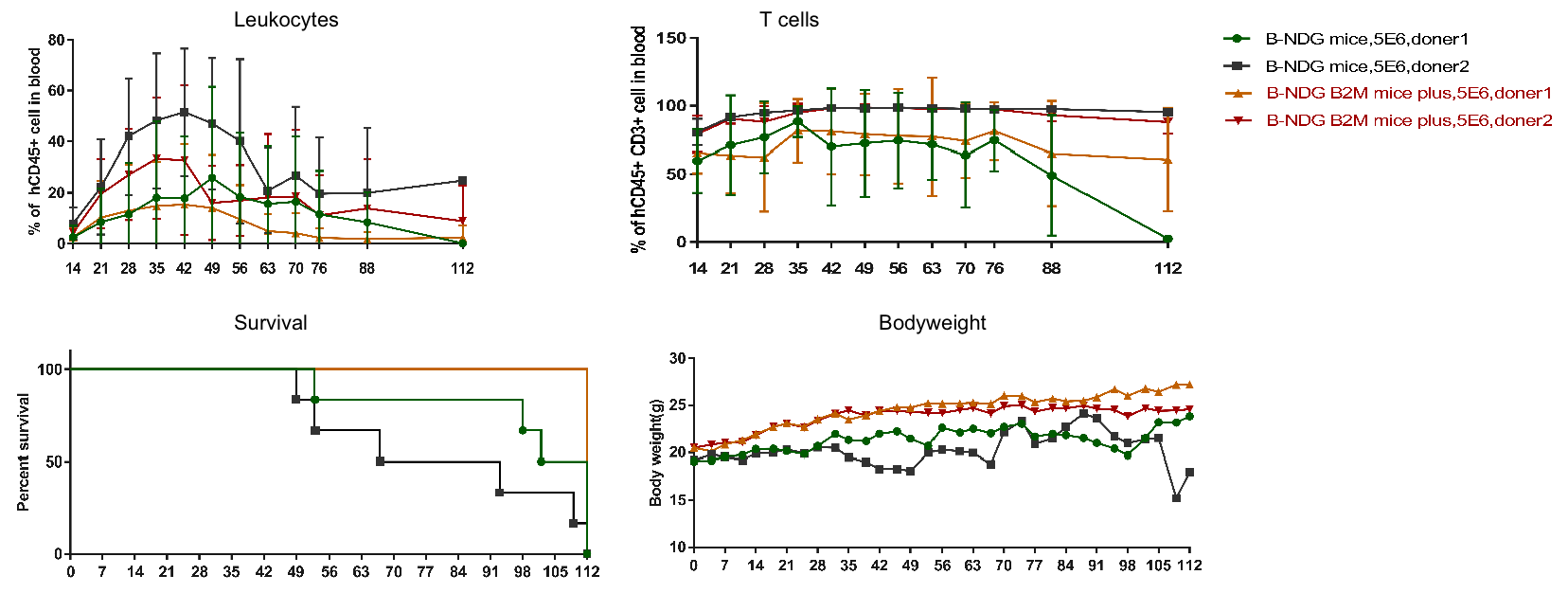
B-NDG B2m KO mice plus and B-NDG mice (female, 7-week-old, n=6) were engrafted with human PBMCs (5×10⁶). Flow cytometric analysis confirmed successful PBMC reconstitution. B-NDG B2m KO mice plus exhibited minimal body-weight loss and extended survival compared with B-NDG mice.
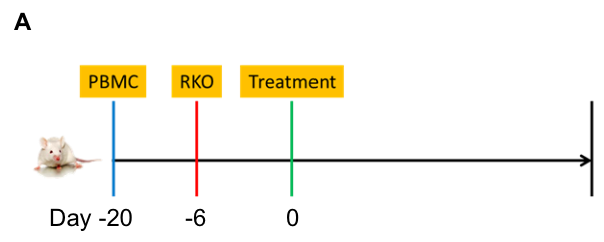
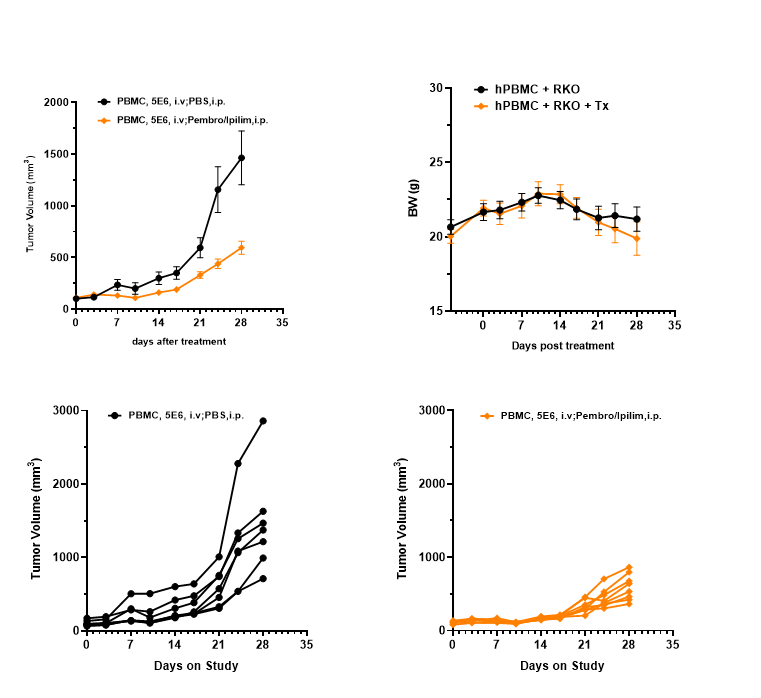
Anti-tumor efficacy in a PBMC–RKO model. Human RKO cells (5×10⁶) were implanted into PBMC-engrafted B-NDG B2m KO Mice Plus (female, 9 weeks old, n=7–8). The combination of an anti–PD-1 antibody (pembrolizumab) and an anti–CTLA-4 antibody (ipilimumab) significantly inhibited tumor growth. Tumor volume and body weight were monitored biweekly.
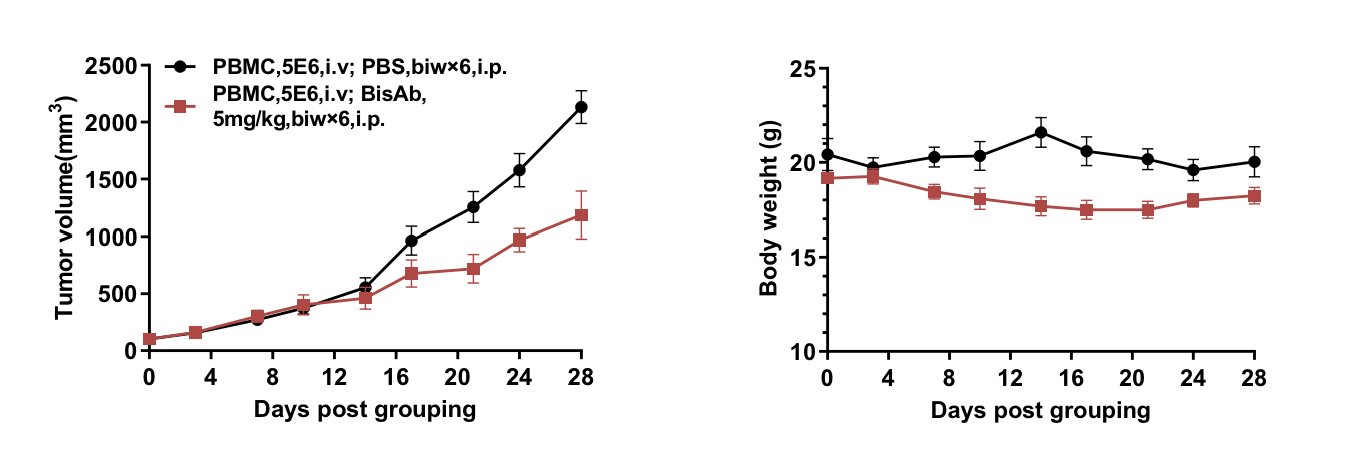
Anti–PD-1×PD-L1 bispecific antibody was tested in PBMC-reconstituted B-NDG B2m KO mice plus bearing RKO tumors (n=6). Treatment began at ~100 mm³ tumor volume. The bispecific antibody significantly reduced tumor growth with stable body weight.
An anti–PD-1×PD-L1 bispecific antibody was tested in PBMC-reconstituted B-NDG B2m KO mice plus bearing RKO tumors (n=6). Treatment began when tumors reached ~100 mm³. The bispecific antibody significantly reduced tumor growth while maintaining stable body weight.
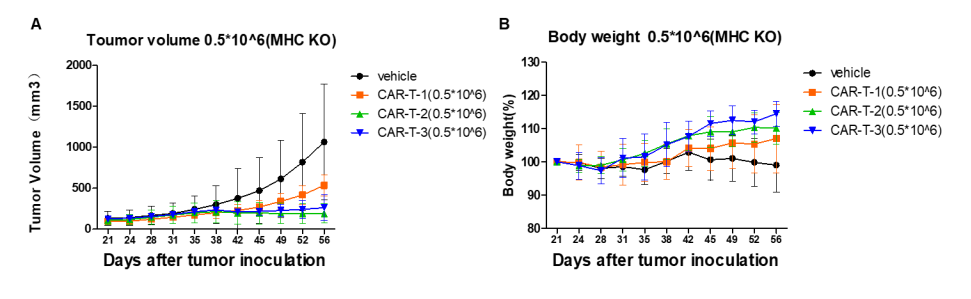
Anti-tumor efficacy of CAR-T cells was evaluated in B-NDG B2m KO mice plus. NCI-H226 cells (1×10⁷) were implanted, and CAR-T cells (5×10⁵) were administered when tumors reached 150 ± 50 mm³. CAR-T treatment significantly inhibited tumor growth, and body weight remained stable.
Q1: What makes B-NDG B2m KO mice plus suitable for PBMC engraftment?
A1: The B2m knockout eliminates MHC-I expression, reducing xenogeneic GvHD and enabling stable human PBMC reconstitution with extended survival.
Q2: Does B2m knockout affect antibody pharmacokinetics?
A2: No. FcRn function is restored via the B2m–Fcgrt fusion gene, allowing normal IgG half-life, essential for therapeutic antibody evaluation.
Q3: Can these mice be used for immuno-oncology studies?
A3: Yes. They support checkpoint inhibitors, bispecific antibodies, T-cell–redirecting therapies, and CAR-T efficacy in CDX models.
Q4: What immune cells reconstitute after PBMC engraftment?
A4: Human CD45⁺ cells, CD4⁺ T cells, CD8⁺ T cells, and Tregs reconstitute effectively, supporting human immune-function studies.
Q5: Do B-NDG B2m KO mice plus reduce GvHD?
A5: Yes. They significantly delay GvHD onset compared with B-NDG mice, extending the experimental window for in vivo studies.
Q6: What are typical applications for this model?
A6: PBMC-based humanized immunity, tumor immunology, GvHD research, antibody PK studies, bispecific antibody testing, and CAR-T evaluation.