
NOD.CB17-Prkdcscid Il2rgtm1Bcgen B2mtm1Bcgen Fcgrttm1(B2m/Fcgrt)Bcgen H2-Ab1tm1Bcgen/Bcgen • 111895

| Product name | B-NDG MHC I/II DKO mice plus |
|---|---|
| Catalog number | 111895 |
| Strain name | NOD.CB17-Prkdcscid Il2rgtm1Bcgen B2mtm1Bcgen Fcgrttm1(B2m/Fcgrt)Bcgen H2-Ab1tm1Bcgen/Bcgen |
| Strain background | B-NDG |
| NCBI gene ID | 12010,14132,16186,19090,14961 (Mouse) |
| Aliases | Ly-m11; beta2m; beta2-m; FcRn; gc; p64; [g]c; CD132; gamma(c); p460; scid; slip; DNAPK; DNPK1; HYRC1; XRCC7; dxnph; DOXNPH; DNAPDcs; DNA-PKcs; IAb; Ia2; Ia-2; Abeta; H-2Ab; H2-Ab; Rmcs1; I-Abeta |
| Application | No MHC class I/II molecule expression; Reduce GVHD and prolong the experimental window period; Improve the accuracy of efficacy verification |
The B-NDG MHC I/II DKO mice plus model was generated by knocking out β2-microglobulin (B2m) as well as both MHC class I (H-2Kᵇ/H-2Dᵇ) and MHC class II (I-Aᵏ) molecules on the NOD background. This produces a highly immunodeficient strain lacking functional T cells, B cells, and NK cells, with complete abolition of murine antigen presentation.
These features significantly reduce xenogeneic graft-versus-host disease (GvHD) induced by human PBMC engraftment and extend the experimental window for long-term human immune-system reconstitution, tumor modeling, and therapeutic antibody evaluation.
This strain enables more stable and longer-lasting human PBMC engraftment, supports robust T-cell reconstitution, and reduces GvHD severity compared with conventional B-NDG or B-NDG B2m KO Mice Plus.
Key Advantages
Validation
Applications
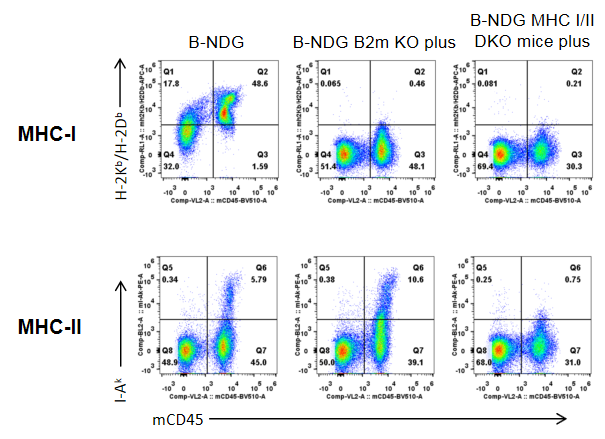
Strain-specific expression of H-2Kb/H-2Db (MHC-I) and I-Ak (MHC-II) was assessed in B-NDG mice, B-NDG B2m KO plus mice, and B-NDG MHC I/II DKO mice plus by flow cytometry. Splenocytes were collected from all three strains and analyzed. Mouse H-2Kb/H-2Db was detectable only in B-NDG mice and absent in both B-NDG B2m KO plus and B-NDG MHC I/II DKO mice plus. Mouse I-Ak was detectable in B-NDG mice and B-NDG B2m KO plus mice, but not in B-NDG MHC I/II DKO mice plus.
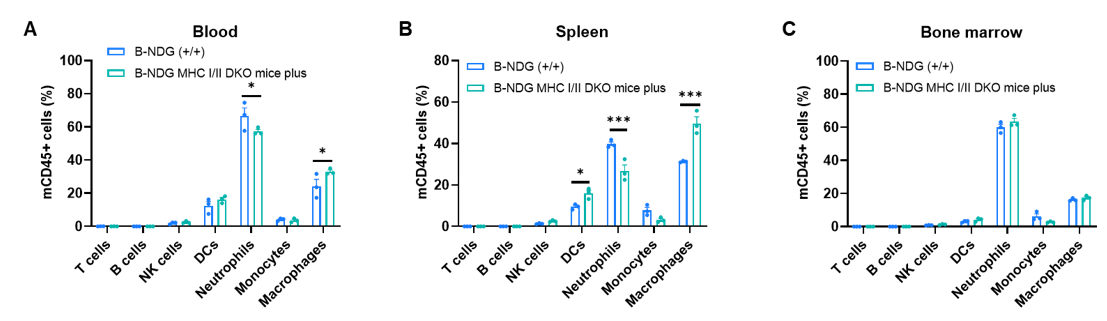
Leukocyte subpopulation frequencies in spleen, blood, and bone marrow were analyzed by flow cytometry in B-NDG mice and B-NDG MHC I/II DKO mice plus (male, 9-week-old, n=3). T cells, B cells, and NK cells were not detectable in any of the examined tissues in either strain. Values are expressed as mean ± SEM. Significance was determined by two-way ANOVA: *P < 0.05, **P < 0.01, ***P < 0.001.
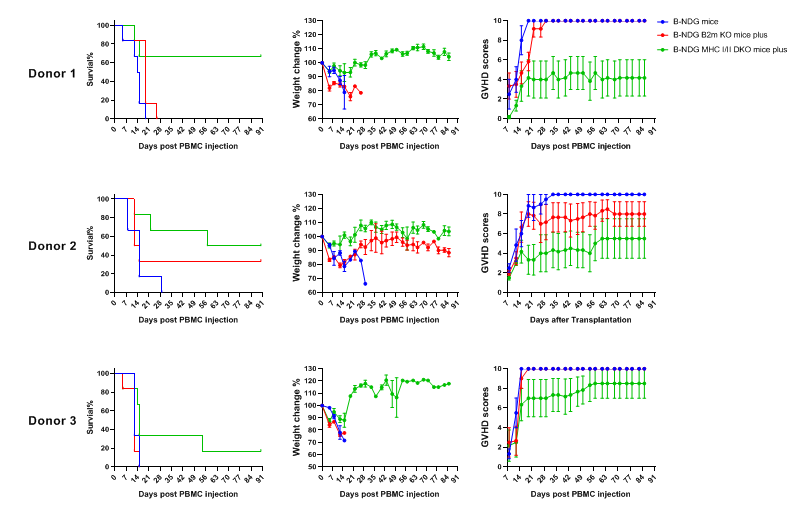
The severity of human PBMC–induced GvHD was compared among B-NDG mice, B-NDG B2m KO mice plus, and B-NDG MHC I/II DKO mice plus. Five-week-old females were intravenously engrafted with human PBMCs (5×10⁶) from three healthy donors (Donors 1-3) on day 0 (n=5). (A) Kaplan–Meier survival analysis. (B) Body weight changes. (C) GvHD clinical scores are assessed twice weekly. Results showed that MHC I/II double knockout significantly prolonged survival and reduced GvHD severity, compared with B-NDG and B-NDG B2m KO mice plus. Therefore, B-NDG MHC I/II DKO mice plus represent a more suitable model for human PBMC engraftment studies. Values are expressed as mean ± SEM.
Comparison of Peripheral Blood Leukocyte Subpopulations After PBMC Engraftment. Female B-NDG mice, B-NDG B2m KO mice plus, and B-NDG MHC I/II DKO mice plus (5 weeks old, n=5) were engrafted intravenously with human PBMCs (5×10⁶) from three donors. Peripheral blood was collected weekly for flow cytometry. The reconstitution levels of all analyzed leukocytes were similar across all strains.
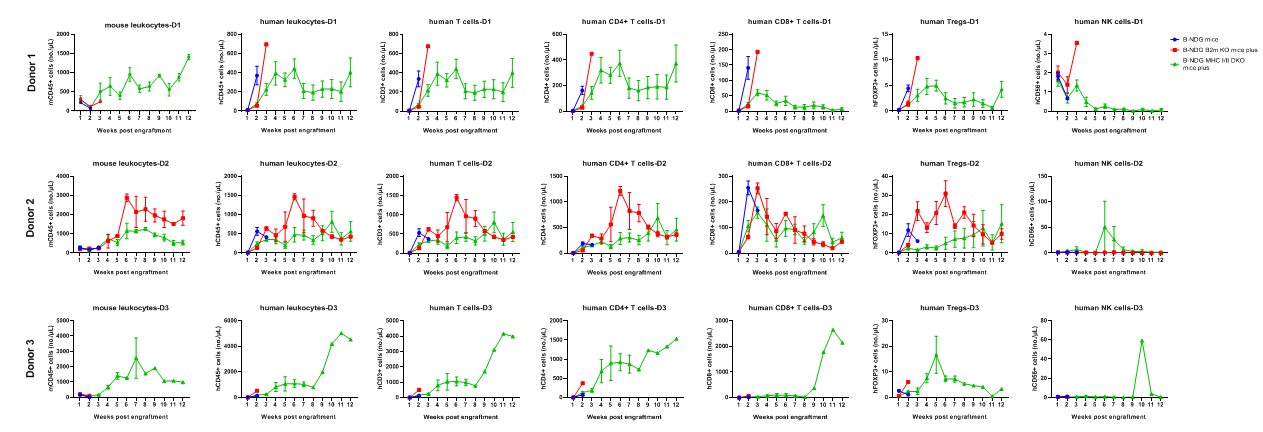
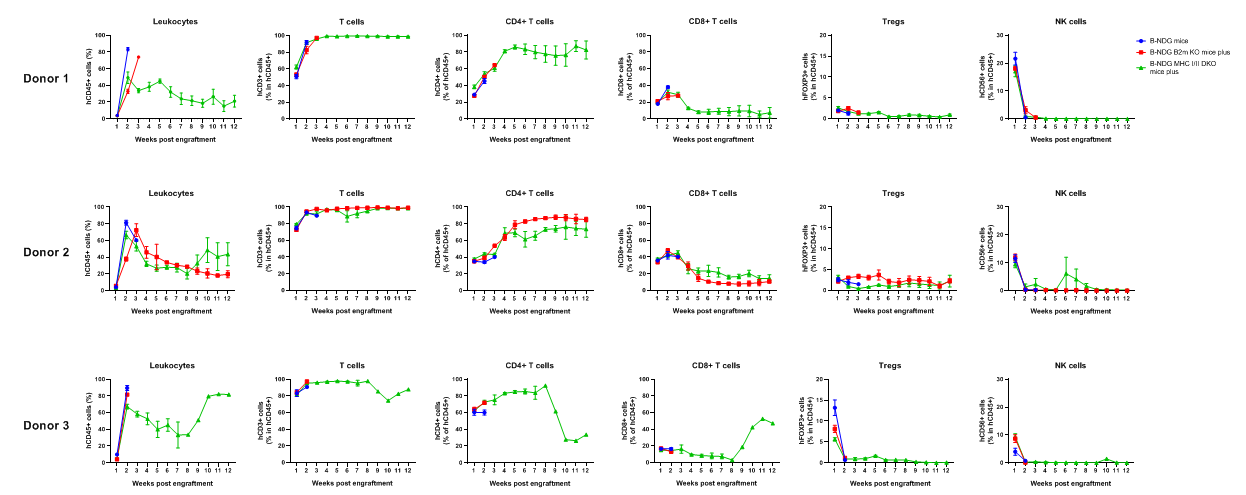
B-NDG mice, B-NDG B2m KO mice plus, and B-NDG MHC I/II DKO mice plus (five-week-old females) were intravenously engrafted with human PBMCs (5×10⁶) from three donors on day 0 (n=6). Peripheral blood was collected weekly for 90 days to assess human immune-cell reconstitution. Various human T-cell subsets were reconstituted in B-NDG MHC I/II DKO mice plus, but the absolute cell numbers were lower than those in B-NDG or B-NDG B2m KO mice plus. Donor 3 displayed the most severe GvHD and produced the highest levels of total reconstituted T cells and CD4⁺ T cells.
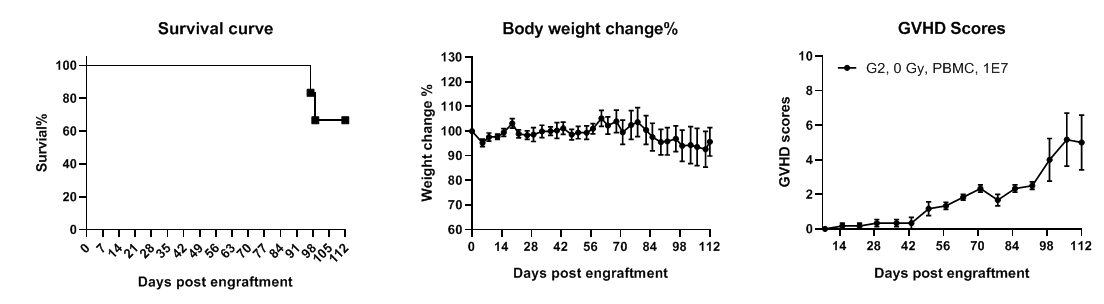
B-NDG MHC I/II DKO mice plus show longer lifespan and reduced GvHD severity with PBMC engraftment. B-NDG MHC I/II DKO mice plus were intravenously injected with human PBMCs (1×10⁷) on day 0 (n=6). Survival was monitored using Kaplan–Meier curves. Body weight was measured twice weekly, and GvHD clinical scoring was performed once weekly. Mice were euthanized upon >20% body-weight loss, with GvHD score recorded as 10. All mice survived up to 96 days, and aside from weight loss, no additional severe GvHD symptoms were observed. Values are expressed as mean ± SEM.
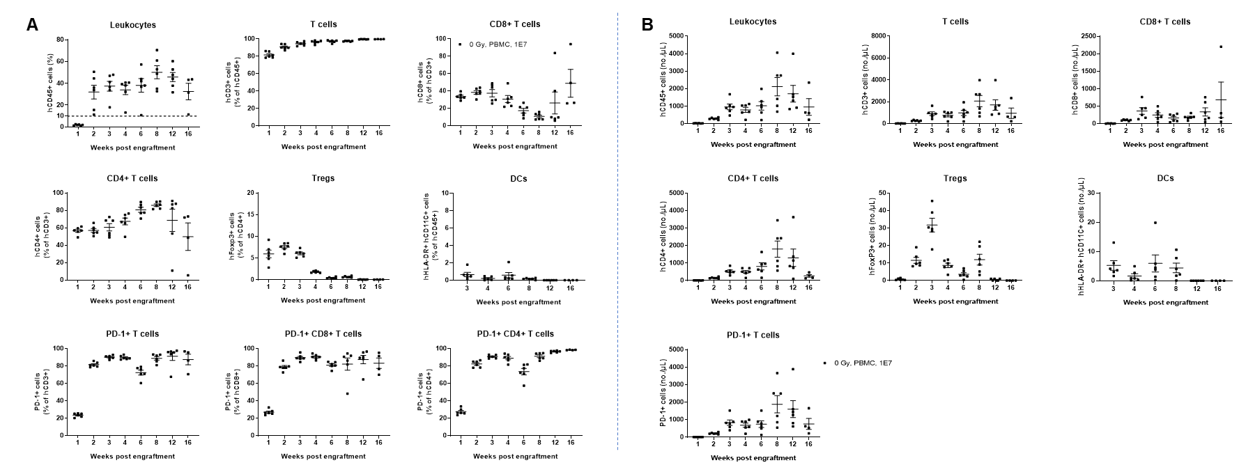
B-NDG MHC I/II DKO mice plus (n=6) were engrafted with human PBMCs (1×10⁷). Peripheral blood was collected weekly, and the study lasted 112 days (16 weeks). (A) Frequency of human immune-cell subpopulations. (B) Absolute cell numbers. CD45⁺ reconstitution increased beginning at week 2 and remained stable through week 16. Human T cells reached >90% at week 2 and nearly 100% by endpoint, including CD4⁺, CD8⁺, and Tregs. A small proportion of DCs was detectable. Human PD-1 was widely expressed on both CD4⁺ and CD8⁺ T cells. These findings demonstrate that B-NDG MHC I/II DKO mice plus are a powerful immunodeficient model for human PBMC-based immune-system reconstitution.
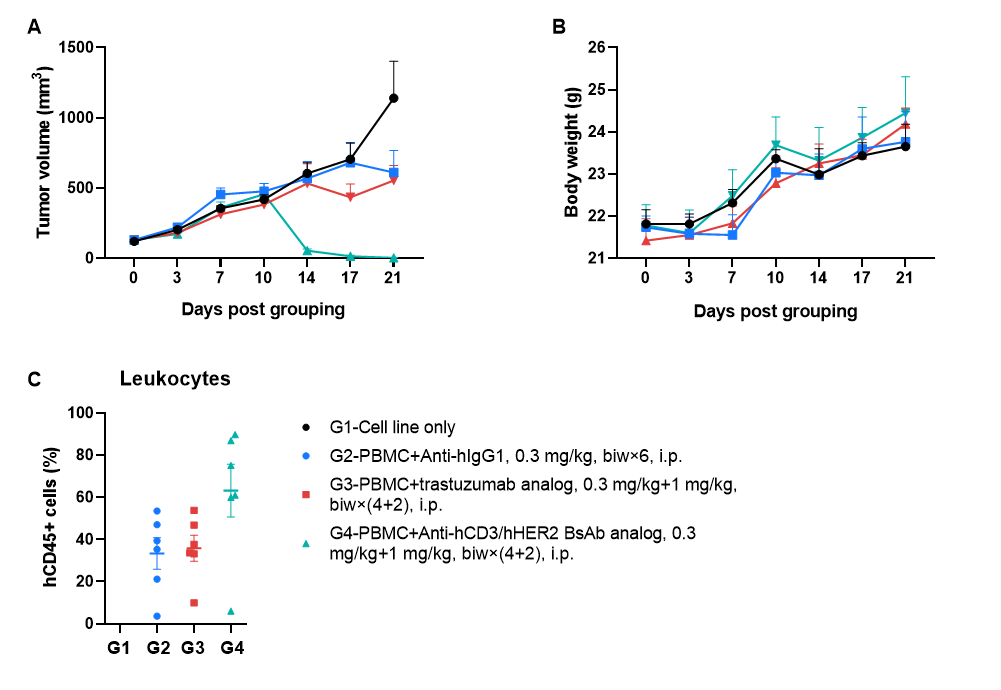
A human gastric cancer CDX model, NCI-N87 (1 × 10⁷), was established in B-NDG MHC I/II DKO mice plus. Human PBMCs (1 × 10⁷) were engrafted intravenously 3 days after tumor inoculation (females, 6–9 weeks old, n = 6). An anti–human CD3 × HER2 bispecific antibody (in-house) and a trastuzumab analog (in-house) were administered intraperitoneally beginning 3 days after tumor inoculation. Treatment began when tumors reached 100 mm³. (A) Tumor volume. (B) Body weight. (C) Frequency of human CD45⁺ peripheral blood cells at endpoint. The anti-human CD3 × HER2 bispecific antibody significantly inhibited tumor growth in a dose-dependent manner.
Q1: What differentiates B-NDG MHC I/II DKO mice plus from other immunodeficient models?
A1: They lack both MHC-I and MHC-II, eliminating murine antigen presentation. This greatly reduces xenogeneic GvHD and supports long-term human PBMC engraftment, making them highly suitable for human immune-system reconstruction and immunotherapy evaluation.
Q2: Why are they ideal for human PBMC engraftment?
A2: The double MHC knockout minimizes host immune activation and enables stable reconstitution of human CD45⁺ cells, CD4⁺ T cells, CD8⁺ T cells, and Tregs for up to 96–112 days. This extended window is valuable for GvHD studies and T-cell–based drug testing.
Q3: Can the model be used for immuno-oncology and bispecific antibody studies?
A3: Yes. These mice support strong human T-cell activation and tumor–immune interactions. In CDX models, bispecific antibodies such as anti-hCD3/hHER2 demonstrate clear, dose-dependent antitumor activity, enabling robust immuno-oncology research.
Q4: Do the mice develop GvHD after PBMC engraftment?
A4: GvHD severity is significantly reduced compared with B-NDG and B-NDG B2m KO mice. Animals maintain longer survival and stable body weight, making the model ideal for longitudinal pharmacology and in vivo therapeutic validation.
Q5: What human immune cells reconstitute in these mice?
A5: They show strong reconstitution of human T-cell subsets (CD4⁺, CD8⁺, Tregs) and detectable dendritic cells (DCs). Human PD-1 expression indicates functional activation. This supports studies in T-cell immunology, checkpoint pathways, and immune-redirecting therapies.
Q6: What are the key research applications?
A6: Main uses include PBMC-derived humanized immune models, GvHD research, CDX tumor models, bispecific antibody testing, checkpoint inhibitor studies, and long-term immune-cell persistence and pharmacology evaluation.