
C57BL/6N-Tnfsf15tm2(TNFSF15)Bcgen/Bcgen • 111997
이 페이지에서
TL1A (TNFSF15), a member of the TNF superfamily, is a costimulatory cytokine involved in T cell activation, inflammation, and mucosal immunity. Dysregulated TL1A signaling has been strongly implicated in inflammatory bowel diseases (IBD) such as Crohn’s disease and ulcerative colitis, as well as other autoimmune conditions.
In TL1A humanized mice, the endogenous murine Tnfsf15 gene is replaced by its human counterpart. Homozygous mice express human TL1A protein while maintaining normal immune cell distribution and physiological parameters. This genetic replacement strategy provides a reliable platform to evaluate anti-human TL1A antibodies and therapeutic agents in vivo.
The TL1A humanized mouse model is an ideal tool for autoimmune and IBD research, enabling TL1A-targeted antibody validation, drug efficacy assessment, and preclinical translational studies.
Key Advantages
Validation
Applications
Gene targeting strategy for B-hTL1A mice. The exons 1-4 of mouse Tl1a gene that encode extracellular domain were replaced by human counterparts in B-hTL1A mice. The genomic region of mouse Tl1a gene that encodes transmembrane domain and cytoplasmic portion was retained. The promoter, 5’UTR and 3’UTR region of the mouse gene were also retained. The chimeric TL1A expression was driven by endogenous mouse Tl1a promoter, while mouse Tl1a gene transcription and translation will be disrupted.
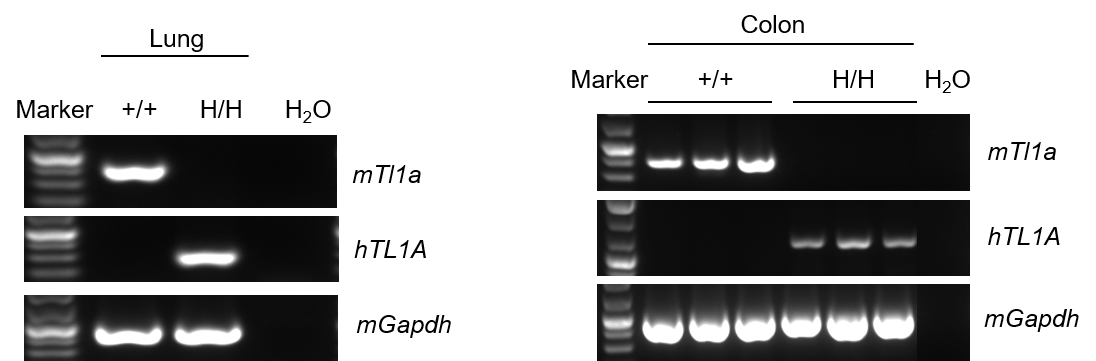
Strain specific analysis of TL1A gene expression in wild-type (WT) mice and B-hTL1A mice by RT-PCR. Mouse Tl1a mRNA was detectable only in lung and colon of wild-type C57BL/6 mice (+/+). Human TL1A mRNA was detectable only in homozygous B-hTL1A mice (H/H) but not in wild-type C57BL/6 mice (+/+) .
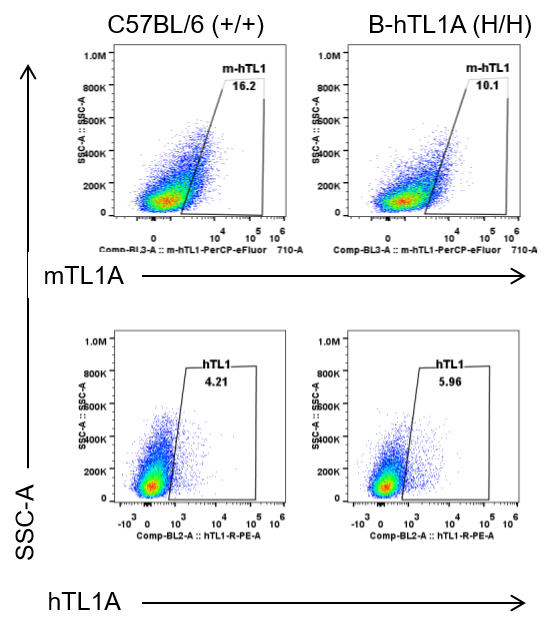
Strain specific TL1A expression analysis in wild-type C57BL/6 mice and homozygous humanized B-hTL1A mice by flow cytometry. Lung endothelial cells were collected from wild-type C57BL/6 mice (+/+) and homozygous B-hTL1A mice (H/H). Protein expression was analyzed with anti-TL1A antibody by flow cytometry. TL1A was detectable in wild-type C57BL/6 mice and homozygous B-hTL1A mice due to the cross-reactivity of antibodies.
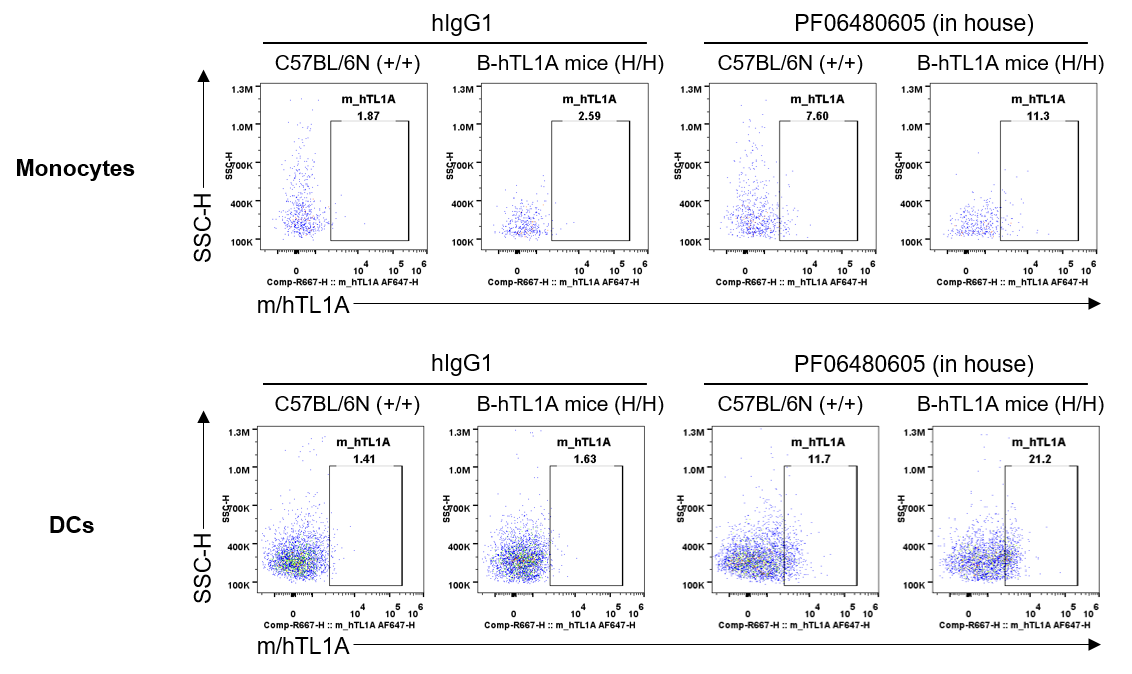
Strain specific TL1A expression analysis in wild-type C57BL/6N mice and homozygous humanized B-hTL1A mice by flow cytometry. Splenocytes were collected from wild-type C57BL/6N mice (+/+) and homozygous B-hTL1A mice(H/H), protein expression was analyzed by flow cytometry with PF06480605 (in house). Mouse/human TL1A was detectable on monocytes and DCs of wild-type C57BL/6N mice and homozygous B-hTL1A mice, as the PF06480605 antibody was cross-reactive between mouse and human.
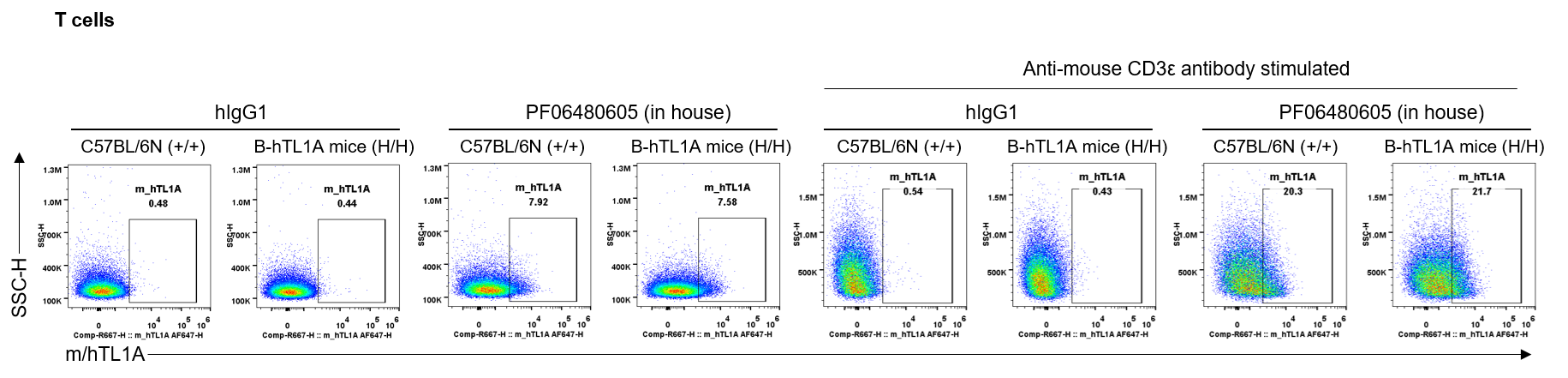
Strain specific TL1A expression analysis in wild-type C57BL/6N mice and homozygous humanized B-hTL1A mice by flow cytometry. Splenocytes were collected from wild-type C57BL/6N mice (+/+) and homozygous B-hTL1A mice(H/H) stimulated with anti-mouse CD3ε antibody in vivo for 24 hrs, protein expression was analyzed by flow cytometry with PF06480605 (in house). Mouse/human TL1A was detectable on T cells of wild-type C57BL/6N mice and homozygous B-hTL1A mice, as the PF06480605 antibody was cross-reactive between mouse and human.
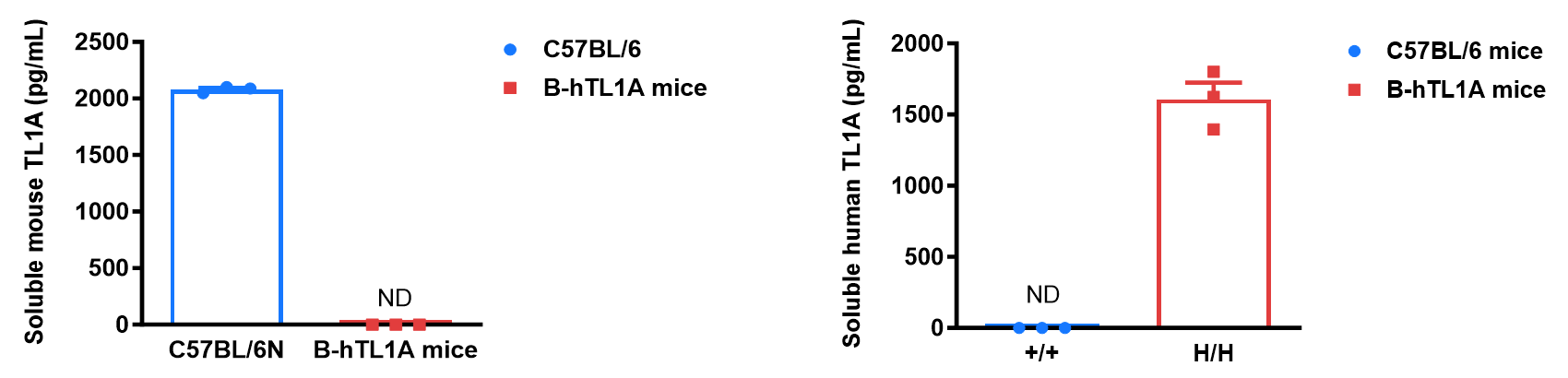
Soluble TL1A expression analysis in B-hTL1A mice by ELISA. Bone marrow derived dendritic cells (BMDCs) were produced by culturing the bone marrow from wild-type C57BL/6 mice (+/+) and homozygous B-hTL1A mice (H/H) (male, 6 weeks-old, n=3), which were stimulated with LPS in vitro. After stimulation, the supernatants were collected and the levels of soluble TL1A were measured using a species-specific mouse and human TL1A ELISA kit. Soluble mouse TL1A was detectable in wild-type C57BL/6 mice. Soluble human TL1A was exclusively detectable in homozygous B-hTL1A mice but not wild-type C57BL/6 mice. Values are expressed as mean ± SEM. ND: not detectable.
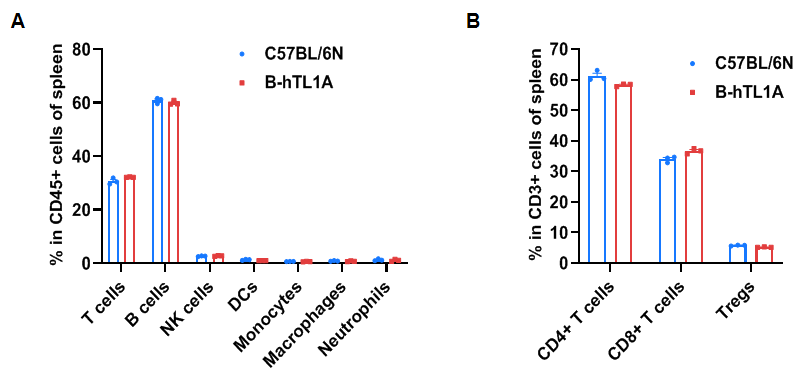
Flow cytometry analysis of splenocytes from wild-type C57BL/6N mice and homozygous TL1A humanized mice (female, 7 weeks old, n=3). Frequencies of T cells, B cells, NK cells, DCs, monocytes, macrophages, neutrophils, CD4⁺ T cells, CD8⁺ T cells, and Tregs in TL1A humanized mice were comparable to those in wild-type mice. Humanization of TL1A does not alter the frequency or distribution of splenic immune cell subsets.
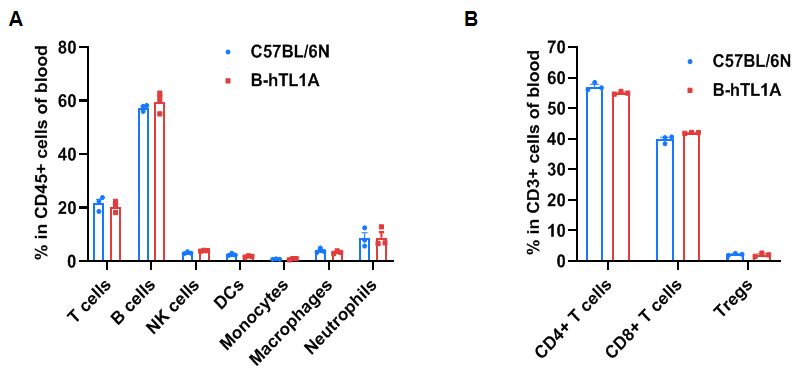
Flow cytometry analysis of blood cells from wild-type C57BL/6N mice and homozygous TL1A humanized mice (female, 7 weeks-old, n=3). The distribution of T cells, B cells, NK cells, DCs, monocytes, macrophages, neutrophils, CD4⁺ T cells, CD8⁺ T cells, and Tregs was similar between TL1A humanized mice and wild-type mice. TL1A humanization does not affect blood leukocyte composition.
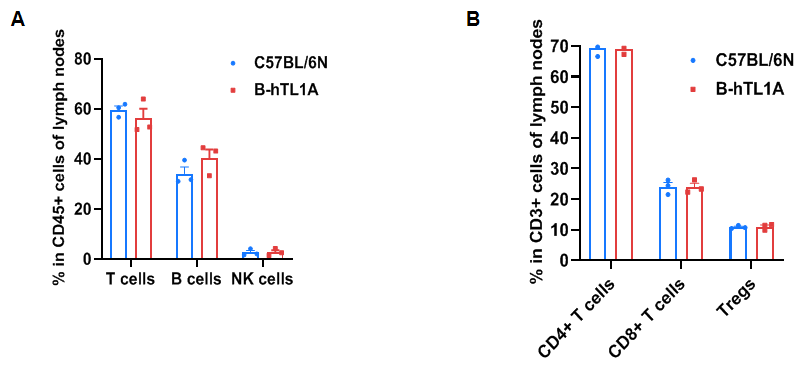
Flow cytometry analysis of leukocytes from wild-type C57BL/6N mice and homozygous TL1A humanized mice (female, 7 weeks old, n=3). Frequencies of T cells, B cells, NK cells, CD4⁺ T cells, CD8⁺ T cells, and Tregs in TL1A humanized mice were comparable to wild-type mice. Humanization of TL1A does not alter leukocyte distribution in lymph nodes.
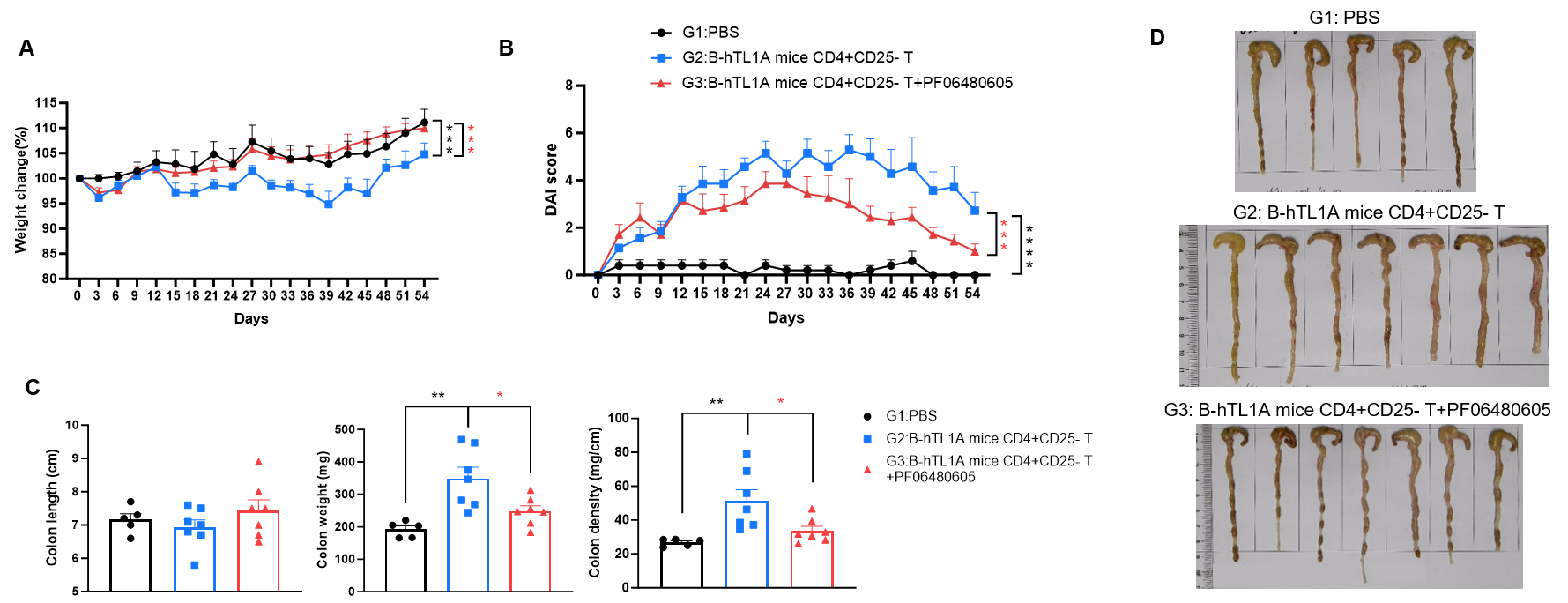
CD4+CD25- T cells were isolated from the spleens of B-hTL1A mice using commercial kits. B-Rag2 KO animals in groups G2-G3 were injected with 6×105 CD4+CD25- T cells, while B-Rag2 KO animals in group G1 were injected with the same volume of PBS. Animals in group G3 were given 25 mg/kg of anti-TL1A antibody PF06480605 (commercially purchased) twice a week. (A) Body weight change. (B) DAI score. (C) Colon index. (D) Colon photo. Administration of anti-TL1A antibody PF06480605 effectively improved T cells-induced colitis, Two-way ANOVA or one-way ANOVA was used for multiple comparisons, with each group compared to group G2. Values are expressed as mean ± SEM. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
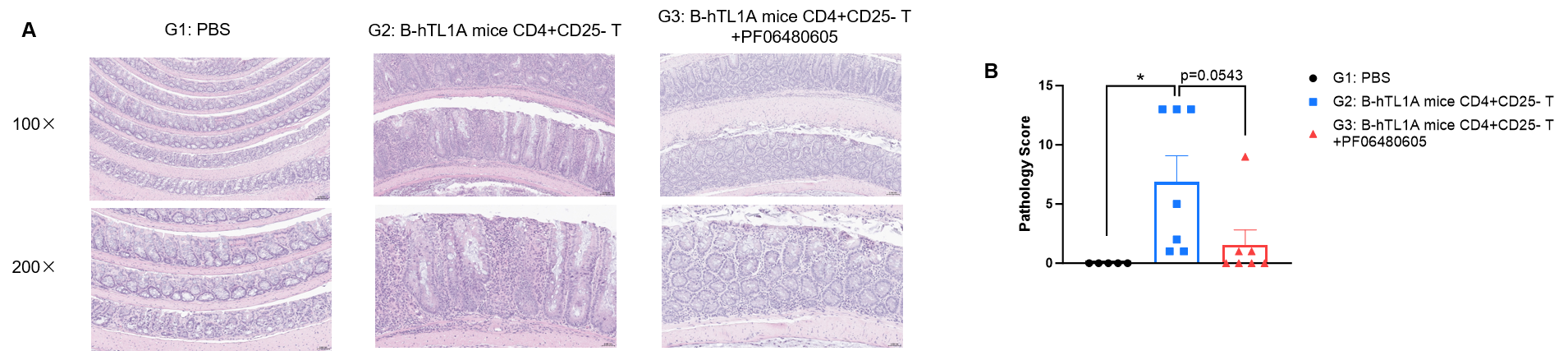
CD4+CD25- T cells were isolated from the spleens of B-hTL1A mice using commercial kits. B-Rag2 KO animals in groups G2-G3 were injected with 6×105 CD4+CD25- T cells, while B-Rag2 KO animals in group G1 were injected with the same volume of PBS. Animals in group G3 were given 25 mg/kg of anti-TL1A antibody PF06480605 (commercially purchased) twice a week. (A) H&E staining of colon tissue. (B) Pathological score. Administration of anti-TL1A antibody PF06480605 improved T cells-induced colitis, Two-way ANOVA or one-way ANOVA was used for multiple comparisons, with each group compared to group G2. Values are expressed as mean ± SEM. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
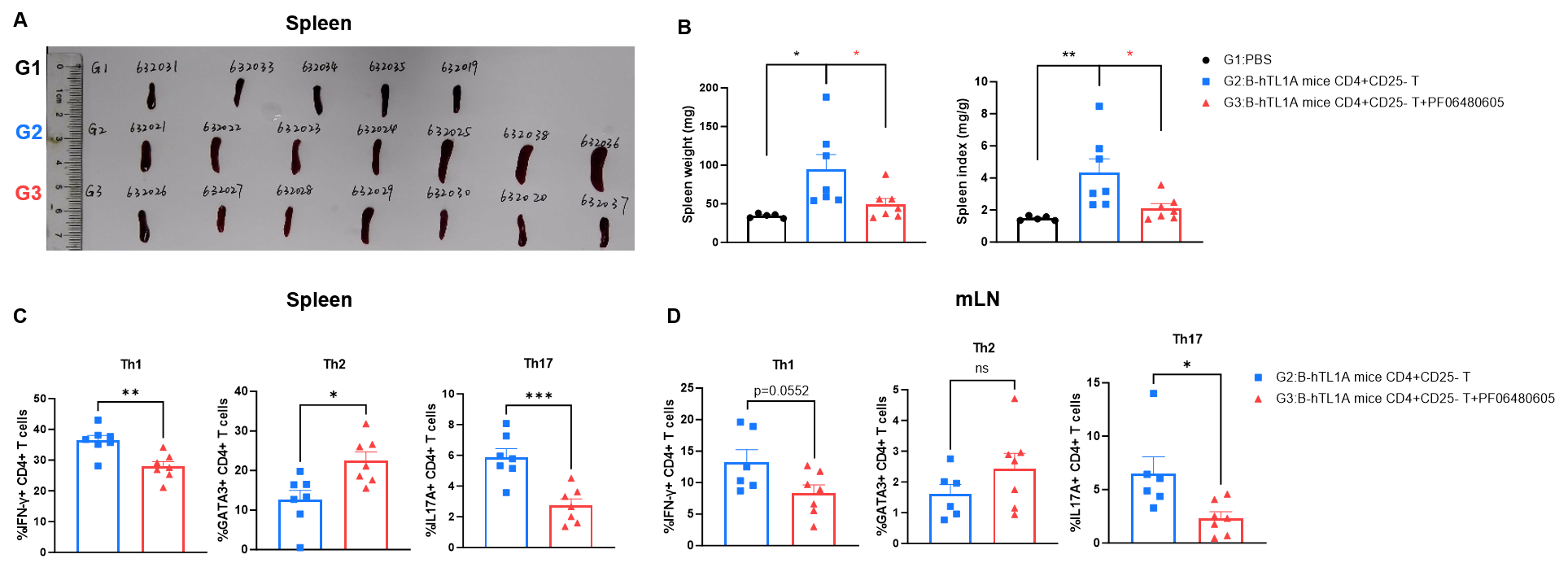
CD4+CD25- T cells were isolated from the spleens of B-hTL1A mice using commercial kits. B-Rag2 KO animals in groups G2-G3 were injected with 6×105 CD4+CD25-T cells, while B-Rag2 KO animals in group G1 were injected with the same volume of PBS. Animals in group G3 were given 25 mg/kg of anti-TL1A antibody PF06480605 (commercially purchased) twice a week. (A) Spleen photo. (B) Spleen index. (C) Percentages of Th1, Th2 and Th17 cells in spleen. (D) Percentages of Th1, Th2 and Th17 cells in mLNs. Administration of anti-TL1A antibody PF06480605 effectively improved T cells-induced colitis, Two-way ANOVA or one-way ANOVA was used for multiple comparisons, with each group compared to group G2. Values are expressed as mean ± SEM. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
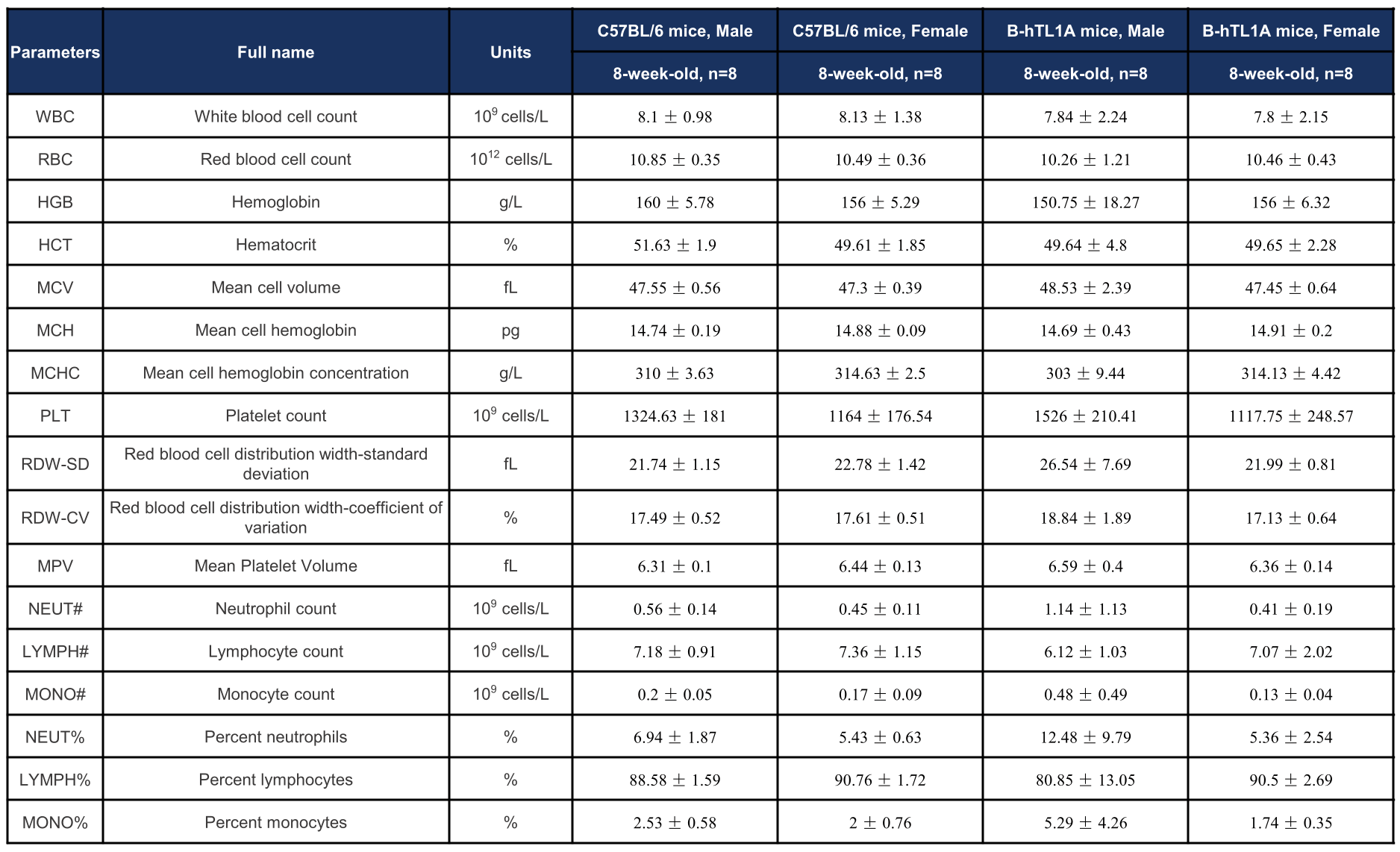
Complete blood count (CBC) of TL1A humanized mice. Values are expressed as mean ± SD.
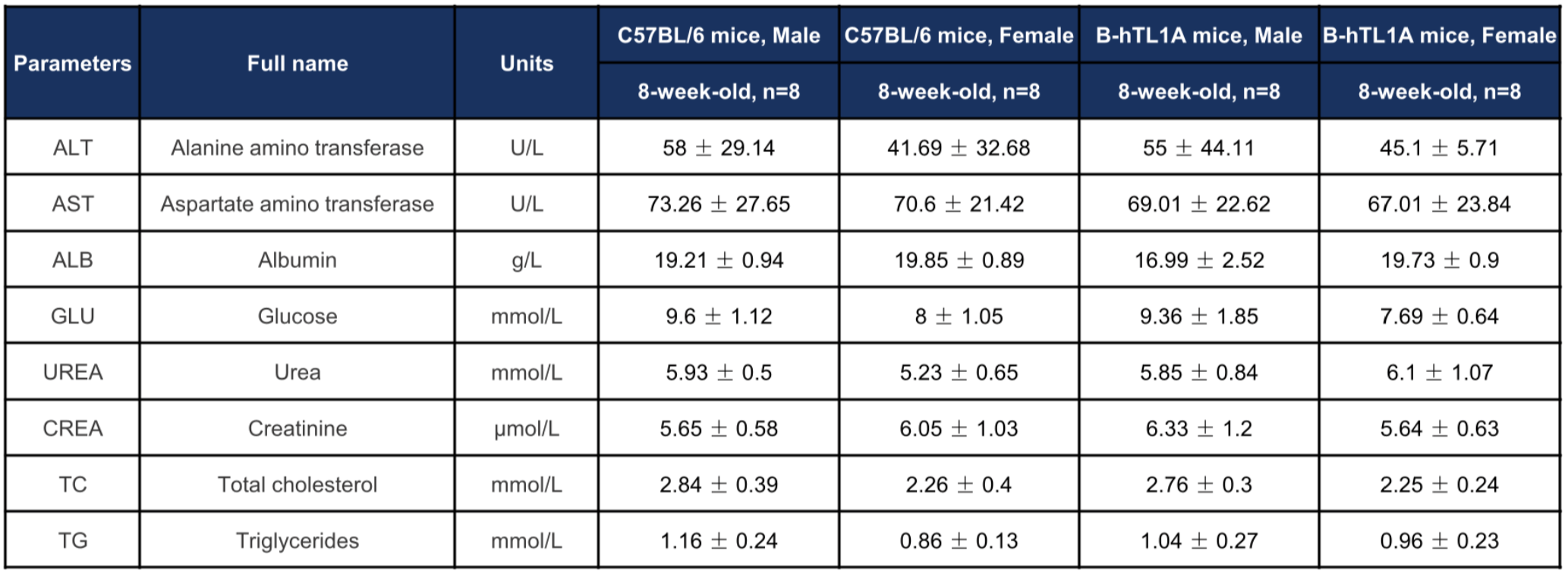
Biochemical profiling of TL1A humanized mice. Values are expressed as mean ± SD.
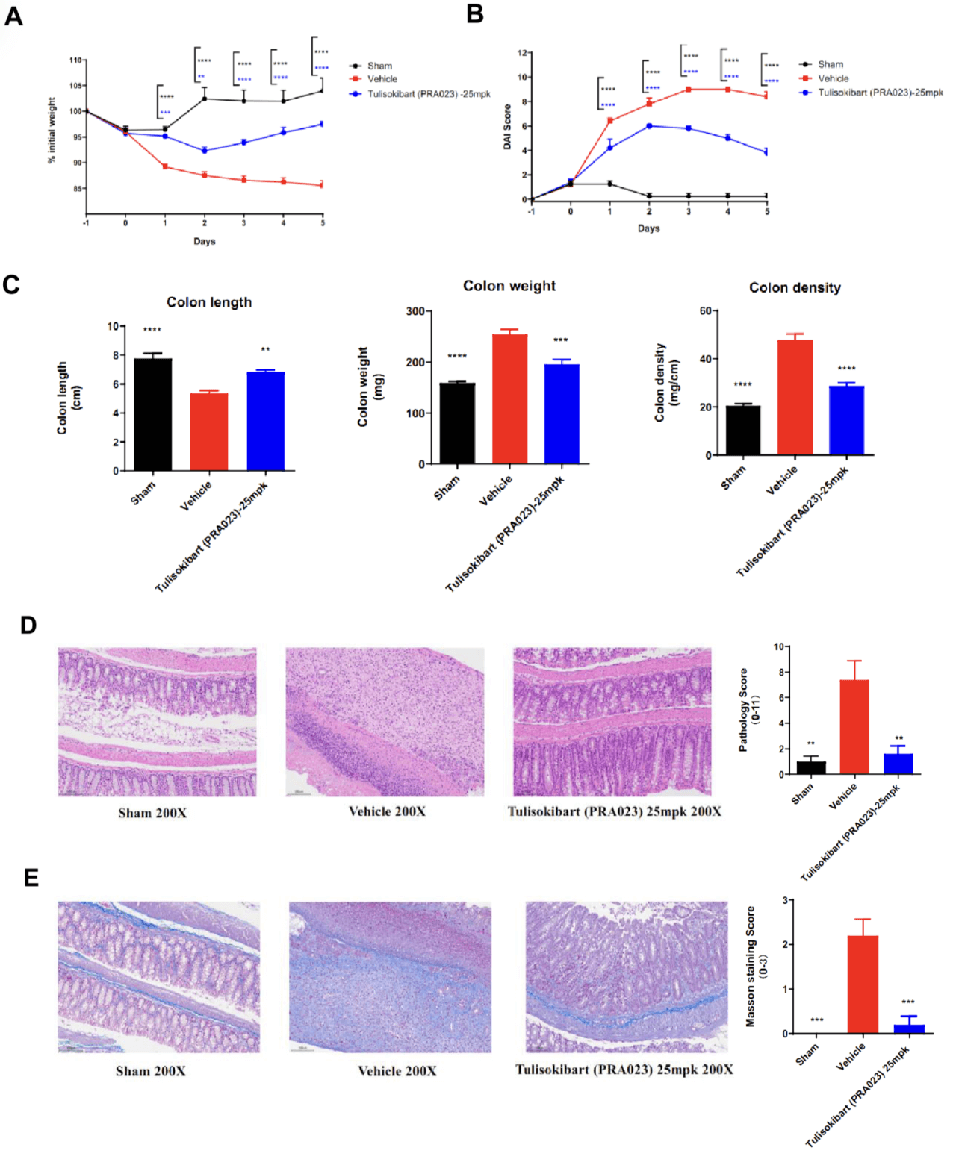
Acute colitis was induced in TL1A humanized mice (female, 8–10 weeks old, n=8) by instillation of TNBS into the colon lumen. Sham controls received PBS. The treatment group received anti-human TL1A antibody Tulisokibart (PRA023, 25 mpk, provided by WuXi AppTec). Body weight, DAI score, colon length and weight, histopathology (H&E, Masson staining) were recorded. Tulisokibart treatment significantly improved TNBS-induced acute colitis. TL1A humanized mice provide a robust in vivo model for evaluating anti-human TL1A antibody efficacy. Values are expressed as mean ± SEM. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 (ANOVA).
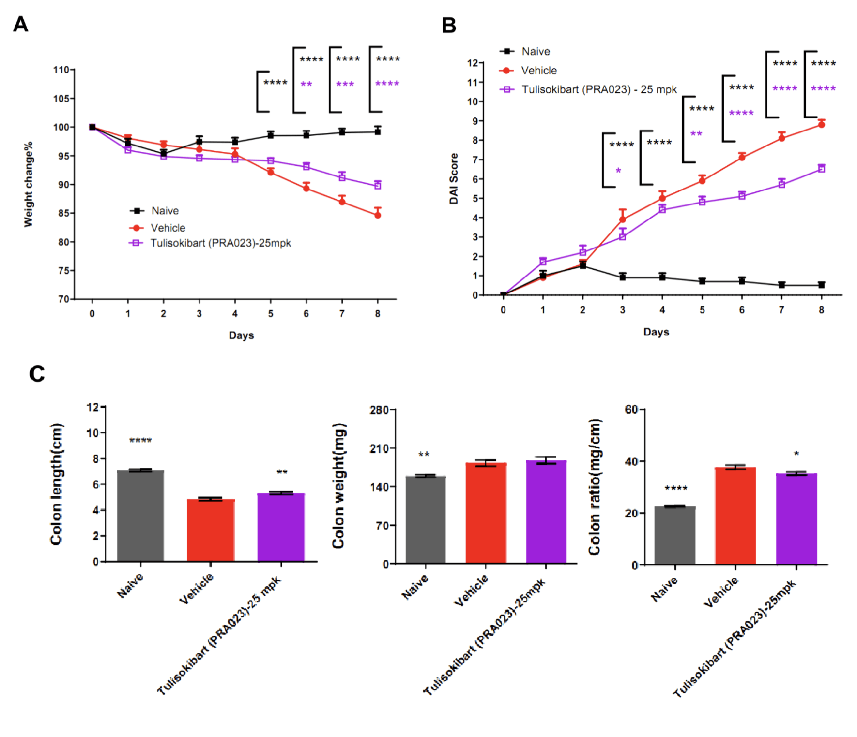
TL1A humanized mice (female, 7–8 weeks old, n=8) were administered DSS in drinking water for 9 consecutive days. The treatment group received Tulisokibart (PRA023, 25 mpk, WuXi AppTec). Body weight, clinical scores (weight loss, stool consistency, blood in stool, total DAI), colon length and weight were recorded. Tulisokibart improved clinical symptoms of DSS-induced colitis. TL1A humanized mice represent a valuable preclinical model for assessing anti-TL1A antibody efficacy. Values are expressed as mean ± SEM. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001 (ANOVA).
When publishing results obtained using this model, please acknowledge as follows: The animal model [TL1A humanized mice (B-hTL1A), Cat# 111997] was purchased from Biocytogen.
Q1: What are TL1A humanized mice?
TL1A humanized mice are genetically engineered models in which the murine Tnfsf15 gene is replaced with the human TNFSF15 gene. These mice express human TL1A protein while maintaining normal immune system function, making them ideal for preclinical research in inflammatory and autoimmune diseases.
Q2: Why are TL1A humanized mice important for inflammatory bowel disease (IBD) research?
Dysregulated TL1A signaling has been strongly implicated in Crohn's disease and ulcerative colitis. TL1A humanized mice enable in vivo validation of anti-human TL1A antibodies and therapeutic candidates, providing a reliable translational model for IBD drug discovery and efficacy testing.
Q3: Can TL1A humanized mice be used for antibody validation?
Yes. TL1A humanized mice support in vivo antibody validation by expressing human TL1A protein, enabling researchers to assess the efficacy, safety, and pharmacokinetics of therapeutic antibodies targeting TL1A.
Q4: How do TL1A humanized mice differ from wild-type mice?
Unlike wild-type mice, which express murine TL1A, TL1A humanized mice express human TL1A exclusively. Importantly, immune cell distribution and hematological/biochemical parameters remain normal, ensuring translational relevance without off-target immunological effects.
Q5: What disease models can be established using TL1A humanized mice?
TL1A humanized mice have been validated in TNBS-induced colitis and DSS-induced colitis models. These models demonstrated improved disease outcomes following treatment with anti-human TL1A antibodies such as Tulisokibart (PRA023), confirming their application in preclinical IBD drug development.